Data source
The data used in this study were obtained from the National Health Information Database (NHID). All individuals in South Korea are included in the National Health Insurance Service (NHIS) system. The NHIS produces and maintains the NHID, which is a public database containing information on healthcare utilization, health screening, sociodemographic factors, and mortality of all Koreans14. The database on healthcare utilization contains information on diagnosis and treatment performed in hospitals. The NHIS uses the Korean Classification of Diseases, which is a classification scheme considered equivalent to the International Classification of Diseases15,16.
Health screening data are obtained from the national health screening program, which is provided to all Koreans over the age of 40 and all employees regardless of age every two years. This includes information on health behaviors obtained from doctor’s counseling and questionnaires filled out by subjects, as well as the results of anthropometric measurements, blood pressure, visual acuity, hearing, and blood and urine tests. Blood pressure was measured after resting for 5 min in a sitting position on the day of the health screening. Blood test items included glucose, total cholesterol, high-density lipoprotein and low-density lipoprotein cholesterol, triglycerides, and creatinine, and tests were performed after 8 h of fasting. The NHIS certifies and regularly conducts quality control evaluation of the hospitals that carry out health screening. The cohort profile was explained in detail in previous review articles15,16,17. In 2009, 66% of people participated in the health screening program18.
Study population
Individuals under the age of 50 who participated in a health screening program in 2009 were included in this study. Among all individuals, 250,392 were excluded due to missing data. A total of 683,044 individuals diagnosed with RA before the health screening examination were also excluded. Finally, 3,537,293 individuals were included in the analysis. Figure 1 shows the eligibility criteria and a flow chart of patient enrollment.
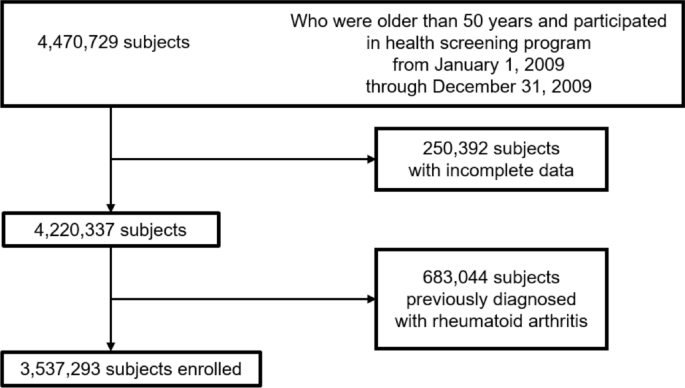
Flow diagram for identifying the study population for incident fractures.
Definition of AMD and visual disability (VD)
Individuals with AMD were identified with a diagnostic code for AMD (International Classification of Diseases 10th revision [ICD-10] code H353) registered by an ophthalmologist in the 1 year prior to the health screening. The definition of AMD as involving a claim with the ICD-10 code for AMD (H353) has also been used in previous epidemiological research on AMD7.
The national disability registration system established by Korean government in 1988 determines welfare benefits according to the type and severity of a disability19. In order to be registered as an individual with a VD, a person must submit documents about their disability diagnosis performed by an ophthalmologist according to detailed criteria (Supplementary Table 2). Individuals with VD were defined as those with a VD certification from the Ministry of Health and Welfare of Korea.
Study outcome and follow-up
The primary outcome was newly diagnosed RA during the study period. Newly diagnosed RA was identified based on the diagnostic code for seropositive RA (ICD-10 code M05) or seronegative RA (ICD-10 code M06) with a prescription of any of the synthetic or biologic disease-modifying anti-rheumatic drugs. A previous study showed that RA was defined by a high positive predictive value and accuracy regarding the ICD-10 code and the prescription of disease-modifying anti-rheumatic drugs in the Korean claims database20. Study individuals were followed from the date of health screening (during 2009) to the date of RA occurrence, death, or December 31, 2019—whichever came first.
Covariates
A number of studies addressing risk factors for RA were reviewed21,22,23. Among the health screening variables, variables that were likely related to RA were included in the multivariable regression model. The selected variables were age, sex, hypertension, diabetes, dyslipidemia, income status, smoking, drinking habits, physical activity, and Charlson comorbidity index (CCI).
Individuals taking anti-hypertensive medications or with systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg were considered to have hypertension. Individuals taking anti-diabetic medications or with fasting glucose ≥ 126 mg/dL were considered to have diabetes mellitus. Individuals taking lipid-lowering medications or with total cholesterol ≥ 240 mg/dL were considered to have dyslipidemia.
Individuals with an income level less than the bottom 20th percentile based on health insurance premiums were defined as low-income residents.
Smoking status was divided into three categories: non-smokers, current smokers, and ex-smokers. Alcohol consumption status was divided into three categories according to average daily alcohol intake: non-drinkers, those with an average intake < 30 g/day, and those with an average intake ≥ 30 g/day. Regular exercise was defined as moderate physical activity carried out for ≥ 30 min ≥ 5 times a week or strenuous physical activity carried out for ≥ 20 min ≥ 3 times a week. Body mass index was calculated as weight in kilograms divided by the square of height in meters (kg/m2).
CCI a well-known and widely used indicator of comorbidity, was calculated as a weighted summed count for 19 diseases. The weights (points) assigned to each condition were as follows: 1 = myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, dementia, chronic pulmonary disease, connective tissue disease, ulcer disease, mild liver disease, or diabetes; 2 = hemiplegia, moderate or severe renal disease, diabetes with end-organ damage, any tumor without metastasis, leukemia, or lymphoma; 3 = moderate or severe liver disease; or 6 = metastatic solid tumor, or acquired immunodeficiency syndrome. These weights were assigned depending on the mortality risk associated with each condition24. CCI score was determined using ICD-10 codes25.
Study protocol approvals
This study adhered to the Declaration of Helsinki, and the study protocol was reviewed and approved by the institutional review board (IRB) of Samsung Medical Center (SMC IRB no. 2022-03-060). The Deliberative Committee of the NHIS approved the conditional use of the database for this study. Informed consent from participants was waived by the IRB of Samsung Medical Center because of the retrospective nature of the study and the analysis used anonymous and public data.
Statistical analysis
Data are described as mean ± standard deviation (SD) and numerical (%) values. Differences between groups (non-AMD vs. AMD or AMD without VD vs. AMD with VD) were investigated using an independent t test for continuous data and the chi-squared test for categorical data.
We analyzed the cumulative incidence of RA in the study population using the Kaplan–Meier curve. Cox regression analysis was conducted to estimate the hazard ratio (HR) and 95% confidence interval (CI) values for the risk of RA development associated with AMD and VD. Variables included in the multivariable adjusted Cox regression model are as follows: model 1 = age and sex; model 2 = model 1 plus income, place, body mass index, smoking habits, alcohol consumption habits, and regular exercise; model 3 = model 2 plus diabetes, hypertension, dyslipidemia, and CCI.
Additional stratified analyses were performed to evaluate the interaction effect of AMD and VD in RA with other variables. Individuals were stratified by age, sex, and the presence of any comorbidity (diabetes, hypertension, or dyslipidemia).
Sensitivity analyses defining RA using only ICD-10 codes were performed.
All statistical analyses were performed using SAS version 9.4 (SAS Institute, Inc., Cary, NC, USA). P < 0.05 was considered to be statistically significant.

