World Health Organization. Obesity and overweight. WHO https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (2025).
Tournadre, A. & Beauger, M. Weight loss affects disease activity and treatment response in inflammatory rheumatic diseases. Jt Bone Spine 91, 105647 (2024).
Google Scholar
Gwinnutt, J. M. et al. Effects of physical exercise and body weight on disease-specific outcomes of people with rheumatic and musculoskeletal diseases (RMDs): systematic reviews and meta-analyses informing the 2021 EULAR recommendations for lifestyle improvements in people with RMDs. RMD Open 8, e002168 (2022).
Google Scholar
Gialouri, C. G., Pappa, M., Evangelatos, G., Nikiphorou, E. & Fragoulis, G. E. Effect of body mass index on treatment response of biologic/targeted-synthetic DMARDs in patients with rheumatoid arthritis, psoriatic arthritis or axial spondyloarthritis. A systematic review. Autoimmun. Rev. 22, 103357 (2023).
Google Scholar
Liu, Y., Hazlewood, G. S., Kaplan, G. G., Eksteen, B. & Barnabe, C. Impact of obesity on remission and disease activity in rheumatoid arthritis: a systematic review and meta-analysis. Arthritis Care Res. 69, 157–165 (2017).
Google Scholar
Eder, L., Thavaneswaran, A., Chandran, V., Cook, R. J. & Gladman, D. D. Obesity is associated with a lower probability of achieving sustained minimal disease activity state among patients with psoriatic arthritis. Ann. Rheum. Dis. 74, 813–817 (2015).
Google Scholar
Leung, Y. Y. et al. Association between obesity and likelihood of remission or low disease activity status in psoriatic arthritis applying index-based and patient-based definitions of remission: a cross-sectional study. RMD Open 9, e003157 (2023).
Google Scholar
Azuaga, A. B., Ramírez, J. & Cañete, J. D. Psoriatic arthritis: pathogenesis and targeted therapies. Int. J. Mol. Sci. 24, 4901 (2023).
Google Scholar
Bakirci, S., Dabague, J., Eder, L., McGonagle, D. & Aydin, S. Z. The role of obesity on inflammation and damage in spondyloarthritis: a systematic literature review on body mass index and imaging. Clin. Exp. Rheumatol. 38, 144–148 (2020).
Google Scholar
Poudel, D., George, M. D. & Baker, J. F. The impact of obesity on disease activity and treatment response in rheumatoid arthritis. Curr. Rheumatol. Rep. 22, 56 (2020).
Google Scholar
Binvignat, M., Sellam, J., Berenbaum, F. & Felson, D. T. The role of obesity and adipose tissue dysfunction in osteoarthritis pain. Nat. Rev. Rheumatol. 20, 565–584 (2024).
Google Scholar
Funck-Brentano, T., Nethander, M., Movérare-Skrtic, S., Richette, P. & Ohlsson, C. Causal factors for knee, hip, and hand osteoarthritis: a Mendelian randomization study in the UK biobank. Arthritis Rheumatol. 71, 1634–1641 (2019).
Google Scholar
Karlsson, T., Hadizadeh, F., Rask-Andersen, M., Johansson, Å & Ek, W. E. Body mass index and the risk of rheumatic disease: linear and nonlinear Mendelian randomization analyses. Arthritis Rheumatol. 75, 2027–2035 (2023).
Google Scholar
Larsson, S. C., Burgess, S. & Michaëlsson, K. Genetic association between adiposity and gout: a Mendelian randomization study. Rheumatology 57, 2145–2148 (2018).
Google Scholar
Cross, M. et al. Global, regional, and national burden of gout, 1990-2020, and projections to 2050: a systematic analysis of the Global Burden of Disease Study 2021. Lancet Rheumatol. 6, e507–e517 (2024).
Google Scholar
Steinmetz, J. D. et al. Global, regional, and national burden of osteoarthritis, 1990-2020 and projections to 2050: a systematic analysis for the Global Burden of Disease Study 2021. Lancet Rheumatol. 5, e508–e522 (2023).
Google Scholar
McCormick, N. et al. Estimation of primary prevention of gout in men through modification of obesity and other key lifestyle factors. JAMA Netw. Open 3, e2027421 (2020).
Google Scholar
Kivimäki, M. et al. Overweight, obesity, and risk of cardiometabolic multimorbidity: pooled analysis of individual-level data for 120 813 adults from 16 cohort studies from the USA and Europe. Lancet Public Health 2, e277–e285 (2017).
Google Scholar
Whitlock, G. et al. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet 373, 1083–1096 (2009).
Google Scholar
Kerola, A. M. et al. All-cause and cause-specific mortality in rheumatoid arthritis, psoriatic arthritis and axial spondyloarthritis: a nationwide registry study. Rheumatology 61, 4656–4666 (2022).
Google Scholar
Vargas-Santos, A. B., Neogi, T., da Rocha Castelar-Pinheiro, G., Kapetanovic, M. C. & Turkiewicz, A. Cause-specific mortality in gout: novel findings of elevated risk of non-cardiovascular-related deaths. Arthritis Rheumatol. 71, 1935–1942 (2019).
Google Scholar
Turkiewicz, A., Kiadaliri, A. A. & Englund, M. Cause-specific mortality in osteoarthritis of peripheral joints. Osteoarthr. Cartil. 27, 848–854 (2019).
Google Scholar
Nurmohamed, M. T., Heslinga, M. & Kitas, G. D. Cardiovascular comorbidity in rheumatic diseases. Nat. Rev. Rheumatol. 11, 693–704 (2015).
Google Scholar
Agca, R. et al. EULAR recommendations for cardiovascular disease risk management in patients with rheumatoid arthritis and other forms of inflammatory joint disorders: 2015/2016 update. Ann. Rheum. Dis. 76, 17–28 (2017).
Google Scholar
Gossec, L. et al. EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2023 update. Ann. Rheum. Dis. 83, 706–719 (2024).
Google Scholar
Moseng, T. et al. EULAR recommendations for the non-pharmacological core management of hip and knee osteoarthritis: 2023 update. Ann. Rheum. Dis. 83, 730–740 (2024).
Google Scholar
Richette, P. et al. 2018 updated European league against rheumatism evidence-based recommendations for the diagnosis of gout. Ann. Rheum. Dis. 79, 31–38 (2020).
Google Scholar
Drucker, D. J. & Holst, J. J. The expanding incretin universe: from basic biology to clinical translation. Diabetologia 66, 1765–1779 (2023).
Google Scholar
Færch, K. et al. GLP-1 response to oral glucose is reduced in prediabetes, screen-detected type 2 diabetes, and obesity and influenced by sex: the ADDITION-PRO study. Diabetes 64, 2513–2525 (2015).
Google Scholar
Drucker, D. J. Efficacy and safety of GLP-1 medicines for type 2 diabetes and obesity. Diabetes Care. 47, 1873–1888 (2024).
Google Scholar
Wharton, S. et al. Daily oral GLP-1 receptor agonist orforglipron for adults with obesity. N. Engl. J. Med. 389, 877–888 (2023).
Google Scholar
Jastreboff, A. M. et al. Triple-hormone-receptor agonist retatrutide for obesity — a phase 2 trial. N. Engl. J. Med. 389, 514–526 (2023).
Google Scholar
Frias, J. P. et al. Efficacy and safety of co-administered once-weekly cagrilintide 2.4 mg with once-weekly semaglutide 2.4 mg in type 2 diabetes: a multicentre, randomised, double-blind, active-controlled, phase 2 trial. Lancet 402, 720–730 (2023).
Google Scholar
Marso, S. P. et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 375, 311–322 (2016).
Google Scholar
Marso, S. P. et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 375, 1834–1844 (2016).
Google Scholar
Wilding, J. P. H. et al. Once-weekly semaglutide in adults with overweight or obesity. N. Engl. J. Med. 384, 989–1002 (2021).
Google Scholar
Davies, M. et al. Semaglutide 2.4 mg once a week in adults with overweight or obesity, and type 2 diabetes (STEP 2): a randomised, double-blind, double-dummy, placebo-controlled, phase 3 trial. Lancet 397, 971–984 (2021).
Google Scholar
Davies, M. J. et al. Efficacy of liraglutide for weight loss among patients with type 2 diabetes: the SCALE diabetes randomized clinical trial. JAMA 314, 687–699 (2015).
Google Scholar
Garvey, W. T. et al. Tirzepatide once weekly for the treatment of obesity in people with type 2 diabetes (SURMOUNT-2): a double-blind, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 402, 613–626 (2023).
Google Scholar
Jastreboff, A. M. et al. Tirzepatide once weekly for the treatment of obesity. N. Engl. J. Med. 387, 205–216 (2022).
Google Scholar
Badve, S. V. et al. Effects of GLP-1 receptor agonists on kidney and cardiovascular disease outcomes: a meta-analysis of randomised controlled trials. Lancet Diabetes Endocrinol. 13, 15–28 (2025).
Google Scholar
Sattar, N. et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of randomised trials. Lancet Diabetes Endocrinol. 9, 653–662 (2021).
Google Scholar
Knop, F. K. et al. Oral semaglutide 50 mg taken once per day in adults with overweight or obesity (OASIS 1): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 402, 705–719 (2023).
Google Scholar
Rosenstock, J. et al. Efficacy and safety of a novel dual GIP and GLP-1 receptor agonist tirzepatide in patients with type 2 diabetes (SURPASS-1): a double-blind, randomised, phase 3 trial. Lancet 398, 143–155 (2021).
Google Scholar
Pi-Sunyer, X. et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N. Engl. J. Med. 373, 11–22 (2015).
Google Scholar
Husain, M. et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 381, 841–851 (2019).
Google Scholar
Drucker, D. J. The GLP-1 journey: from discovery science to therapeutic impact. J. Clin. Invest. 134, e175634 (2024).
Google Scholar
Newsome, P. N. et al. A placebo-controlled trial of subcutaneous semaglutide in nonalcoholic steatohepatitis. N. Engl. J. Med. 384, 1113–1124 (2021).
Google Scholar
Armstrong, M. J. et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet 387, 679–690 (2016).
Google Scholar
Loomba, R. et al. Tirzepatide for metabolic dysfunction-associated steatohepatitis with liver fibrosis. N. Engl. J. Med. 391, 299–310 (2024).
Google Scholar
Yabut, J. M. & Drucker, D. J. Glucagon-like peptide-1 receptor-based therapeutics for metabolic liver disease. Endocr. Rev. 44, 14–32 (2023).
Google Scholar
Daousi, C., Pinkney, J. H., Cleator, J., Wilding, J. P. & Ranganath, L. R. Acute peripheral administration of synthetic human GLP-1 (7-36 amide) decreases circulating IL-6 in obese patients with type 2 diabetes mellitus: a potential role for GLP-1 in modulation of the diabetic pro-inflammatory state? Regul. Pept. 183, 54–61 (2013).
Google Scholar
Chaudhuri, A. et al. Exenatide exerts a potent antiinflammatory effect. J. Clin. Endocrinol. Metab. 97, 198–207 (2012).
Google Scholar
Karacabeyli, D. & Lacaille, D. Glucagon-like peptide 1 receptor agonists in patients with inflammatory arthritis or psoriasis: a scoping review. J. Clin. Rheumatol. 30, 26–31 (2024).
Google Scholar
Meurot, C. et al. Liraglutide, a glucagon-like peptide 1 receptor agonist, exerts analgesic, anti-inflammatory and anti-degradative actions in osteoarthritis. Sci. Rep. 12, 1567 (2022).
Google Scholar
Zhang, X. et al. Liraglutide, a glucagon-like peptide-1 receptor agonist, ameliorates inflammation and apoptosis via inhibition of receptor for advanced glycation end products signaling in AGEs induced chondrocytes. BMC Musculoskelet. Disord. 25, 601 (2024).
Google Scholar
Li, H., Chen, J., Li, B. & Fang, X. The protective effects of dulaglutide against advanced glycation end products (AGEs)-induced degradation of type II collagen and aggrecan in human SW1353 chondrocytes. Chem. Biol. Interact. 322, 108968 (2020).
Google Scholar
Li, X., Jia, F., Zhu, Z. & Huang, L. Lixisenatide attenuates advanced glycation end products (AGEs)-induced degradation of extracellular matrix in human primary chondrocytes. Artif. Cell Nanomed. Biotechnol. 47, 1256–1264 (2019).
Google Scholar
Chen, J. et al. Glucagon-like peptide-1 receptor regulates endoplasmic reticulum stress-induced apoptosis and the associated inflammatory response in chondrocytes and the progression of osteoarthritis in rat. Cell Death Dis. 9, 212 (2018).
Google Scholar
Que, Q. et al. The GLP-1 agonist, liraglutide, ameliorates inflammation through the activation of the PKA/CREB pathway in a rat model of knee osteoarthritis. J. Inflamm. 16, 13 (2019).
Google Scholar
Tong, C. et al. The protective effects of exenatide against AGEs-induced articular matrix degradation in human primary chondrocytes. Am. J. Transl. Res. 11, 2081–2089 (2019).
Google Scholar
Mei, J., Sun, J., Wu, J. & Zheng, X. Liraglutide suppresses TNF-α-induced degradation of extracellular matrix in human chondrocytes: a therapeutic implication in osteoarthritis. Am. J. Transl. Res. 11, 4800–4808 (2019).
Google Scholar
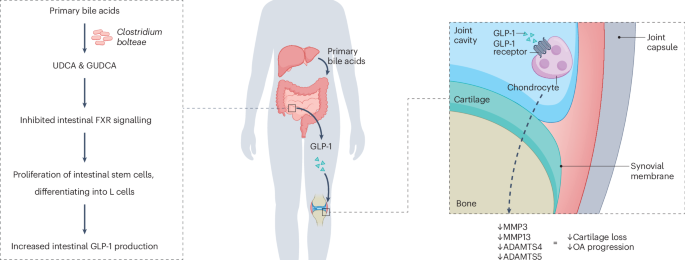
Yang, Y. et al. Osteoarthritis treatment via the GLP-1–mediated gut-joint axis targets intestinal FXR signaling. Science 388, eadt0548 (2025).
Google Scholar
Yang, J., Wang, Z. & Zhang, X. GLP-1 receptor agonist impairs keratinocytes inflammatory signals by activating AMPK. Exp. Mol. Pathol. 107, 124–128 (2019).
Google Scholar
Tao, Y. et al. Exenatide ameliorates inflammatory response in human rheumatoid arthritis fibroblast-like synoviocytes. IUBMB Life 71, 969–977 (2019).
Google Scholar
Zheng, W., Pan, H., Wei, L., Gao, F. & Lin, X. Dulaglutide mitigates inflammatory response in fibroblast-like synoviocytes. Int. Immunopharmacol. 74, 105649 (2019).
Google Scholar
Du, X. et al. The protective effects of lixisenatide against inflammatory response in human rheumatoid arthritis fibroblast-like synoviocytes. Int. Immunopharmacol. 75, 105732 (2019).
Google Scholar
Liu, T., Zhang, L., Joo, D. & Sun, S.-C. NF-κB signaling in inflammation. Signal. Transduct. Target. Ther. 2, 17023 (2017).
Google Scholar
Salminen, A., Hyttinen, J. M. & Kaarniranta, K. AMP-activated protein kinase inhibits NF-κB signaling and inflammation: impact on healthspan and lifespan. J. Mol. Med. 89, 667–676 (2011).
Google Scholar
Baser, O., Isenman, L., Baser, S. & Samayoa, G. Impact of semaglutide on osteoarthritis risk in patients with obesity: a retrospective cohort study. Obes. Sci. Pract. 10, e762 (2024).
Google Scholar
Baser, O. et al. The impact of approved anti-obesity medications on osteoarthritis. Expert. Opin. Pharmacother. 25, 1565–1573 (2024).
Google Scholar
Lavu, M. S. et al. The five-year incidence of progression to osteoarthritis and total joint arthroplasty in patients prescribed glucagon-like peptide 1 receptor agonists. J. Arthroplast. 39, 2433–2439.e1 (2024).
Google Scholar
Porto, J. R. et al. The impact of contemporary glucagon-like peptide-1 receptor agonists on the onset, severity, and conversion to arthroplasty in hip and knee osteoarthritis. Orthop. J. Sports Med. 13, 23259671241297157 (2025).
Google Scholar
Hunter, D. J. & Bierma-Zeinstra, S. Osteoarthritis. Lancet 393, 1745–1759 (2019).
Google Scholar
Yau, M. S. et al. Validation of knee osteoarthritis case identification algorithms in a large electronic health record database. Osteoarthr. Cartil. Open 4, 100229 (2022).
Google Scholar
Rahman, M. M., Kopec, J. A., Goldsmith, C. H., Anis, A. H. & Cibere, J. Validation of administrative osteoarthritis diagnosis using a clinical and radiological population-based cohort. Int. J. Rheumatol. 2016, 6475318 (2016).
Google Scholar
Shahid, A. et al. Comparison of weight loss interventions in overweight and obese adults with knee osteoarthritis: a systematic review and network meta-analysis of randomized trials. Osteoarthritis Cartilage 33, 518–529 (2024).
Google Scholar
Bliddal, H. et al. Once-weekly semaglutide in persons with obesity and knee osteoarthritis. N. Engl. J. Med. 391, 1573–1583 (2024).
Google Scholar
Garvey, W. T. et al. American Association of Clinical Endocrinologists and American College of Endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity. Endocr. Pract. 22, 1–203 (2016).
Google Scholar
Clement, N. D. et al. What is the minimum clinically important difference for the WOMAC index after TKA? Clin. Orthop. Relat. Res. 476, 2005–2014 (2018).
Google Scholar
Silva, M. D. C., Perriman, D. M., Fearon, A. M., Couldrick, J. M. & Scarvell, J. M. Minimal important change and difference for knee osteoarthritis outcome measurement tools after non-surgical interventions: a systematic review. BMJ Open 13, e063026 (2023).
Google Scholar
Garvey, W. T. et al. Two-year effects of semaglutide in adults with overweight or obesity: the STEP 5 trial. Nat. Med. 28, 2083–2091 (2022).
Google Scholar
Gudbergsen, H. et al. Liraglutide after diet-induced weight loss for pain and weight control in knee osteoarthritis: a randomized controlled trial. Am. J. Clin. Nutr. 113, 314–323 (2021).
Google Scholar
Roos, E. M. & Lohmander, L. S. The knee injury and osteoarthritis outcome score (KOOS): from joint injury to osteoarthritis. Health Qual. Life Outcomes 1, 64 (2003).
Google Scholar
Zhu, H. et al. Glucagon-like peptide-1 receptor agonists as a disease-modifying therapy for knee osteoarthritis mediated by weight loss: findings from the Shanghai osteoarthritis cohort. Ann. Rheum. Dis. 82, 1218–1226 (2023).
Google Scholar
Samajdar, S. S. et al. Dual effects of dulaglutide on glycemic control and knee osteoarthritis pain in elderly patients with type 2 diabetes. Pain Manag. 14, 365–373 (2024).
Google Scholar
Park, D. et al. Association between knee osteoarthritis and the risk of cardiovascular disease and the synergistic adverse effects of lack of exercise. Sci. Rep. 13, 2777 (2023).
Google Scholar
Hall, A. J., Stubbs, B., Mamas, M. A., Myint, P. K. & Smith, T. O. Association between osteoarthritis and cardiovascular disease: systematic review and meta-analysis. Eur. J. Prev. Cardiol. 23, 938–946 (2016).
Google Scholar
Gao, K. et al. Is chronic kidney disease associated with osteoarthritis? The United States National Health and Nutrition Examination Survey 2011–2020. BMC Nephrol. 25, 236 (2024).
Google Scholar
Nüesch, E. et al. All cause and disease specific mortality in patients with knee or hip osteoarthritis: population based cohort study. BMJ 342, d1165 (2011).
Google Scholar
Kendzerska, T. et al. The longitudinal relationship between hand, hip and knee osteoarthritis and cardiovascular events: a population-based cohort study. Osteoarthr. Cartil. 25, 1771–1780 (2017).
Google Scholar
Wang, A., Shi, W., Zhang, N., Tang, H. & Feng, X. Newer glucose-lowering drugs and risk of gout: a network meta-analysis of randomized outcomes trials. Clin. Ther. 46, 851–854 (2024).
Google Scholar
Preston, F. G. et al. SGLT2 inhibitors, but not GLP-1 receptor agonists, reduce incidence of gout in people living with type 2 diabetes across the therapeutic spectrum. Clin. Ther. 46, 835–840 (2024).
Google Scholar
Wood, D. T., Waterbury, N. V. & Lund, B. C. Sodium glucose cotransporter 2 inhibitors and gout risk: a sequence symmetry analysis. Clin. Rheumatol. 42, 2469–2475 (2023).
Google Scholar
Tesfaye, H. et al. Empagliflozin and risk of incident gout: analysis from the EMPagliflozin comparative effectiveness and safEty (EMPRISE) cohort study. J. Gen. Intern. Med. 39, 1870–1879 (2024).
Google Scholar
Lund, L. C., Højlund, M., Henriksen, D. P., Hallas, J. & Kristensen, K. B. Sodium-glucose cotransporter-2 inhibitors and the risk of gout: a Danish population based cohort study and symmetry analysis. Pharmacoepidemiol. Drug Saf. 30, 1391–1395 (2021).
Google Scholar
Fralick, M., Chen, S. K., Patorno, E. & Kim, S. C. Assessing the risk for gout with sodium-glucose cotransporter-2 inhibitors in patients with type 2 diabetes: a population-based cohort study. Ann. Intern. Med. 172, 186–194 (2020).
Google Scholar
Shah, B. R. et al. Pharmacologic glycemic management of type 2 diabetes in adults — 2024 update. Can. J. Diabetes 48, 415–424 (2024).
Google Scholar
Pereira, M. J. & Eriksson, J. W. Emerging role of SGLT-2 inhibitors for the treatment of obesity. Drugs 79, 219–230 (2019).
Google Scholar
Packer, M. Hyperuricemia and gout reduction by SGLT2 inhibitors in diabetes and heart failure. J. Am. Coll. Cardiol. 83, 371–381 (2024).
Google Scholar
Tonneijck, L. et al. Effect of immediate and prolonged GLP-1 receptor agonist administration on uric acid and kidney clearance: post-hoc analyses of four clinical trials. Diabetes Obes. Metab. 20, 1235–1245 (2018).
Google Scholar
Najafi, S., Bahrami, M., Butler, A. E. & Sahebkar, A. The effect of glucagon-like peptide-1 receptor agonists on serum uric acid concentration: a systematic review and meta-analysis. Br. J. Clin. Pharmacol. 88, 3627–3637 (2022).
Google Scholar
Nielsen, S. M. et al. Weight loss for overweight and obese individuals with gout: a systematic review of longitudinal studies. Ann. Rheum. Dis. 76, 1870 (2017).
Google Scholar
Wei, J. et al. Gout flares and mortality after sodium-glucose cotransporter-2 inhibitor treatment for gout and type 2 diabetes. JAMA Netw. Open 6, e2330885 (2023).
Google Scholar
McCormick, N. et al. Comparative effectiveness of sodium-glucose cotransporter-2 inhibitors for recurrent nephrolithiasis among patients with pre-existing nephrolithiasis or gout: target trial emulation studies. BMJ 387, e080035 (2024).
Google Scholar
Zhu, Y., Pandya, B. J. & Choi, H. K. Comorbidities of gout and hyperuricemia in the US general population: NHANES 2007-2008. Am. J. Med. 125, 679–687.e1 (2012).
Google Scholar
Lincoff, A. M. et al. Semaglutide and cardiovascular outcomes in obesity without diabetes. N. Engl. J. Med. 389, 2221–2232 (2023).
Google Scholar
Kikkawa, K. et al. Long-acting glucagon-like peptide-1 receptor agonist-induced rheumatoid arthritis in a patient with type 2 diabetes mellitus. Dubai Diabetes Endocrinol. J. 27, 114–117 (2021).
Google Scholar
Ambrosio, M. L. et al. GLP-1 receptor agonist-induced polyarthritis: a case report. Acta Diabetol. 51, 673–674 (2014).
Google Scholar
Nassar, M., Nassar, O., Abosheaishaa, H. & Misra, A. Comparative outcomes of systemic diseases in people with type 2 diabetes, or obesity alone treated with and without GLP-1 receptor agonists: a retrospective cohort study from the global collaborative network. J. Endocrinol. Invest. 48, 483–497 (2025).
Google Scholar
Ohno, T., Aune, D. & Heath, A. K. Adiposity and the risk of rheumatoid arthritis: a systematic review and meta-analysis of cohort studies. Sci. Rep. 10, 16006 (2020).
Google Scholar
Eriksson, J. K. et al. Incidence of rheumatoid arthritis in Sweden: a nationwide population-based assessment of incidence, its determinants, and treatment penetration. Arthritis Care Res. 65, 870–878 (2013).
Google Scholar
Myasoedova, E., Crowson, C. S., Kremers, H. M., Therneau, T. M. & Gabriel, S. E. Is the incidence of rheumatoid arthritis rising? Results from Olmsted County, Minnesota, 1955-2007. Arthritis Rheum. 62, 1576–1582 (2010).
Google Scholar
Sullivan, C. et al. Treatment with the glucagon-like peptide-1 analogue liraglutide is associated with amelioration of disease activity in a prospective cohort study of patients with inflammatory arthritis. Arthritis Rheumatol. 65, S630–S631 (2013).
Hogan, A. E. & O’Shea, D. Glucagon-like peptide-1: a key regulator of innate immune function with clinical efficacy in a range of inflammatory diseases. Diabetes 61, A25 (2012).
Nicolau, J., Nadal, A., Ros, I. & Masmiquel, L. Effects of liraglutide among patients with psoriatic arthritis and obesity. Reumatol. Clin. 21, 501809 (2025).
Google Scholar
Faurschou, A. et al. Lack of effect of the glucagon-like peptide-1 receptor agonist liraglutide on psoriasis in glucose-tolerant patients — a randomized placebo-controlled trial. J. Eur. Acad. Dermatol. Venereol. 29, 555–559 (2015).
Google Scholar
Lin, L. et al. Glucagon-like peptide-1 receptor agonist liraglutide therapy for psoriasis patients with type 2 diabetes: a randomized-controlled trial. J. Dermatol. Treat. 33, 1428–1434 (2022).
Google Scholar
Petković-Dabić, J. et al. Effects of semaglutide treatment on psoriatic lesions in obese patients with type 2 diabetes mellitus: an open-label, randomized clinical trial. Biomolecules 15, 46 (2025).
Google Scholar
Kanie, T. et al. Dipeptidyl peptidase-4 inhibitors, glucagon-like peptide 1 receptor agonists and sodium-glucose co-transporter-2 inhibitors for people with cardiovascular disease: a network meta-analysis. Cochrane Database Syst. Rev. 10, CD013650 (2021).
Google Scholar
Karacabeyli, D. et al. Mortality and major adverse cardiovascular events after glucagon-like peptide-1 receptor agonist initiation in patients with immune-mediated inflammatory diseases and type 2 diabetes: a population-based study. PLoS ONE 19, e0308533 (2024).
Google Scholar
Ruff, C. T. et al. Subcutaneous infusion of exenatide and cardiovascular outcomes in type 2 diabetes: a non-inferiority randomized controlled trial. Nat. Med. 28, 89–95 (2022).
Google Scholar
Gilbert, M. P. & Pratley, R. E. GLP-1 analogs and DPP-4 inhibitors in type 2 diabetes therapy: review of head-to-head clinical trials. Front. Endocrinol. 11, 178 (2020).
Google Scholar
Food and Drug Administration. Highlights of prescribing information: OZEMPIC (semaglutide) injection, for subcutaneous use. FDA https://www.accessdata.fda.gov/drugsatfda_docs/label/2025/209637s025lbl.pdf (2025).
Bjerre Knudsen, L. et al. Glucagon-like peptide-1 receptor agonists activate rodent thyroid C-cells causing calcitonin release and C-cell proliferation. Endocrinology 151, 1473–1486 (2010).
Google Scholar
Drummond, R. F., Seif, K. E. & Reece, E. A. Glucagon-like peptide-1 receptor agonist use in pregnancy: a review. Am. J. Obstet. Gynecol. 232, 17–25 (2025).
Google Scholar
Dong, S. & Sun, C. Can glucagon-like peptide-1 receptor agonists cause acute kidney injury? An analytical study based on post-marketing approval pharmacovigilance data. Front. Endocrinol. 13, 1032199 (2022).
Google Scholar
Keller, J. et al. Effect of exenatide on cholecystokinin-induced gallbladder emptying in fasting healthy subjects. Regul. Pept. 179, 77–83 (2012).
Google Scholar
Oprea, A. D. et al. Perioperative management of patients taking glucagon-like peptide 1 receptor agonists: Society for Perioperative Assessment and Quality Improvement (SPAQI) multidisciplinary consensus statement. Br. J. Anaesth. 135, 48–78 (2025).
Google Scholar
Kornelius, E., Huang, J.-Y., Lo, S.-C., Huang, C.-N. & Yang, Y.-S. The risk of depression, anxiety, and suicidal behavior in patients with obesity on glucagon like peptide-1 receptor agonist therapy. Sci. Rep. 14, 24433 (2024).
Google Scholar
Guirguis, A. et al. Exploring the association between suicidal thoughts, self-injury, and GLP-1 receptor agonists in weight loss treatments: insights from pharmacovigilance measures and unmasking analysis. Eur. Neuropsychopharmacol. 82, 82–91 (2024).
Google Scholar
Schoretsanitis, G., Weiler, S., Barbui, C., Raschi, E. & Gastaldon, C. Disproportionality analysis from World Health Organization data on semaglutide, liraglutide, and suicidality. JAMA Netw. Open 7, e2423385 (2024).
Google Scholar
McIntyre, R. S. et al. Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) and suicidality: a replication study using reports to the World Health Organization pharmacovigilance database (VigiBase®). J. Affect. Disord. 369, 922–927 (2025).
Google Scholar
Ueda, P. et al. GLP-1 receptor agonist use and risk of suicide death. JAMA Intern. Med. 184, 1301–1312 (2024).
Google Scholar
Hurtado, I., Robles, C., Peiró, S., García-Sempere, A. & Sanfélix-Gimeno, G. Association of glucagon-like peptide-1 receptor agonists with suicidal ideation and self-injury in individuals with diabetes and obesity: a propensity-weighted, population-based cohort study. Diabetologia 67, 2471–2480 (2024).
Google Scholar
Tang, H. et al. Glucagon-like peptide-1 receptor agonists and risk for suicidal ideation and behaviors in U.S. older adults with type 2 diabetes. Ann. Intern. Med. 177, 1004–1015 (2024).
Google Scholar
De Giorgi, R. et al. 12-month neurological and psychiatric outcomes of semaglutide use for type 2 diabetes: a propensity-score matched cohort study. eClinicalMedicine 74, 102726 (2024).
Google Scholar
Kim, T. H. et al. Association between glucagon-like peptide-1 receptor agonists and risk of suicidality: a comprehensive analysis of the global pharmacovigilance database. Diabetes Obes. Metab. 26, 5183–5191 (2024).
Google Scholar
Wang, W. et al. Association of semaglutide with risk of suicidal ideation in a real-world cohort. Nat. Med. 30, 168–176 (2024).
Google Scholar
Food and Drug Administration. Update on FDA’s ongoing evaluation of reports of suicidal thoughts or actions in patients taking a certain type of medicines approved for type 2 diabetes and obesity. FDA https://www.fda.gov/drugs/drug-safety-and-availability/update-fdas-ongoing-evaluation-reports-suicidal-thoughts-or-actions-patients-taking-certain-type (2024).
Blake, T., Gullick, N. J., Hutchinson, C. E. & Barber, T. M. Psoriatic disease and body composition: a systematic review and narrative synthesis. PLoS ONE 15, e0237598 (2020).
Google Scholar
Letarouilly, J. G., Flipo, R. M., Cortet, B., Tournadre, A. & Paccou, J. Body composition in patients with rheumatoid arthritis: a narrative literature review. Ther. Adv. Musculoskelet. Dis. 13, 1759720X211015006 (2021).
Google Scholar
Al Refaie, A. et al. Glucagon-like peptide-1 receptor agonists (GLP-1RAs) for the treatment of type 2 diabetes mellitus: friends or foes to bone health? A narrative review of clinical studies. Endocrine 89, 30–38 (2025).
Google Scholar
Zhang, Y. et al. Association of glucagon-like peptide-1 receptor agonists use with fracture risk in type 2 diabetes: a meta-analysis of randomized controlled trials. Bone 192, 117338 (2025).
Google Scholar
Hansen, M. S. et al. Once-weekly semaglutide versus placebo in adults with increased fracture risk: a randomised, double-blinded, two-centre, phase 2 trial. eClinicalMedicine 72, 102624 (2024).
Google Scholar
Jensen, S. B. K. et al. Bone health after exercise alone, GLP-1 receptor agonist treatment, or combination treatment: a secondary analysis of a randomized clinical trial. JAMA Netw. Open 7, e2416775 (2024).
Google Scholar
Turicchi, J. et al. Associations between the proportion of fat-free mass loss during weight loss, changes in appetite, and subsequent weight change: results from a randomized 2-stage dietary intervention trial. Am. J. Clin. Nutr. 111, 536–544 (2020).
Google Scholar
Heymsfield, S. B., Gonzalez, M. C., Shen, W., Redman, L. & Thomas, D. Weight loss composition is one-fourth fat-free mass: a critical review and critique of this widely cited rule. Obes. Rev. 15, 310–321 (2014).
Google Scholar
Ross, R. et al. Canadian 24-hour movement guidelines for adults aged 18-64 years and adults aged 65 years or older: an integration of physical activity, sedentary behaviour, and sleep. Appl. Physiol. Nutr. Metab. 45, S57–s102 (2020).
Google Scholar
Tinsley, G. M. & Heymsfield, S. B. Fundamental body composition principles provide context for fat-free and skeletal muscle loss with GLP-1 RA treatments. J. Endocr. Soc. 8, bvae164 (2024).
Google Scholar
Leidy, H. J. et al. The role of protein in weight loss and maintenance. Am. J. Clin. Nutr. 101, 1320s–1329s (2015).
Google Scholar
Heymsfield, S. B. et al. Effect of bimagrumab vs placebo on body fat mass among adults with type 2 diabetes and obesity: a phase 2 randomized clinical trial. JAMA Netw. Open 4, e2033457 (2021).
Google Scholar
Rooks, D. et al. Bimagrumab vs optimized standard of care for treatment of sarcopenia in community-dwelling older adults: a randomized clinical trial. JAMA Netw. Open 3, e2020836 (2020).
Google Scholar
Rubino, D. et al. Effect of continued weekly subcutaneous semaglutide vs placebo on weight loss maintenance in adults with overweight or obesity: the STEP 4 randomized clinical trial. JAMA 325, 1414–1425 (2021).
Google Scholar
Aronne, L. J. et al. Continued treatment with tirzepatide for maintenance of weight reduction in adults with obesity: the SURMOUNT-4 randomized clinical trial. JAMA 331, 38–48 (2024).
Google Scholar
Manne-Goehler, J., Teufel, F. & Venter, W. D. F. GLP-1 receptor agonists and the path to sustainable obesity care. JAMA Intern. Med. 185, 8–10 (2025).
Google Scholar
Cengiz, A., Wu, C. C. & Lawley, S. D. Alternative dosing regimens of GLP-1 receptor agonists may reduce costs and maintain weight loss efficacy. Diabetes Obes. Metab. 27, 2251–2258 (2025).
Google Scholar
Paddu, N. U., Lawrence, B., Wong, S., Poon, S. J. & Srivastava, G. Weight maintenance on cost-effective antiobesity medications after 1 year of GLP-1 receptor agonist therapy: a real-world study. Obesity 32, 2255–2263 (2024).
Google Scholar
Jensen, S. B. K. et al. Healthy weight loss maintenance with exercise, GLP-1 receptor agonist, or both combined followed by one year without treatment: a post-treatment analysis of a randomised placebo-controlled trial. EClinicalMedicine 69, 102475 (2024).
Google Scholar
Falkentoft, A. C. et al. Impact of socioeconomic position on initiation of SGLT-2 inhibitors or GLP-1 receptor agonists in patients with type 2 diabetes — a Danish nationwide observational study. Lancet Reg. Health Eur. 14, 100308 (2022).
Google Scholar
Eberly, L. A. et al. Racial, ethnic, and socioeconomic inequities in glucagon-like peptide-1 receptor agonist use among patients with diabetes in the US. JAMA Health Forum 2, e214182 (2021).
Google Scholar
Bastick, A. N., Belo, J. N., Runhaar, J. & Bierma-Zeinstra, S. M. What are the prognostic factors for radiographic progression of knee osteoarthritis? A meta-analysis. Clin. Orthop. Relat. Res. 473, 2969–2989 (2015).
Google Scholar
Lewis, G. N., Rice, D. A., McNair, P. J. & Kluger, M. Predictors of persistent pain after total knee arthroplasty: a systematic review and meta-analysis. Br. J. Anaesth. 114, 551–561 (2015).
Google Scholar
Ashoorion, V. et al. Predictors of persistent post-surgical pain following total knee arthroplasty: a systematic review and meta-analysis of observational studies. Pain Med. 24, 369–381 (2022).
Google Scholar
Nguyen, U. D. et al. Obesity paradox in recurrent attacks of gout in observational studies: clarification and remedy. Arthritis Care Res. 69, 561–566 (2017).
Google Scholar
Bajpai, R. et al. Onset of comorbidities and flare patterns within pre-existing morbidity clusters in people with gout: 5-year primary care cohort study. Rheumatology 61, 407–412 (2021).
Google Scholar
Rothenbacher, D., Primatesta, P., Ferreira, A., Cea-Soriano, L. & Rodríguez, L. A. G. Frequency and risk factors of gout flares in a large population-based cohort of incident gout. Rheumatology 50, 973–981 (2011).
Google Scholar
Cheng, Z. et al. Obesity reduces the urate-lowering efficacy among patients with primary gout: a prospective cohort study. Rheumatology 64, 3500–3508 (2025).
Google Scholar
Mu, Z. et al. Predictors of poor response to urate-lowering therapy in patients with gout and hyperuricemia: a post-hoc analysis of a multicenter randomized trial. Clin. Rheumatol. 38, 3511–3519 (2019).
Google Scholar
Sun, W. et al. Predictors of inadequate serum urate response to low-dose febuxostat in male patients with gout. J. Inflamm. Res. 17, 2657–2668 (2024).
Google Scholar
Latourte, A. et al. Dyslipidemia, alcohol consumption, and obesity as main factors associated with poor control of urate levels in patients receiving urate-lowering therapy. Arthritis Care Res. 70, 918–924 (2018).
Google Scholar
Xie, W., Huang, H., Deng, X., Gao, D. & Zhang, Z. Modifiable lifestyle and environmental factors associated with onset of psoriatic arthritis in patients with psoriasis: a systematic review and meta-analysis of observational studies. J. Am. Acad. Dermatol. 84, 701–711 (2021).
Google Scholar
Guglielmi, G. et al. The role of DXA in sarcopenia. Aging Clin. Exp. Res. 28, 1047–1060 (2016).
Google Scholar
Wang, Z. et al. Hydration of fat-free body mass: review and critique of a classic body-composition constant. Am. J. Clin. Nutr. 69, 833–841 (1999).
Google Scholar
Wang, J. & Pierson, R. N. Jr. Disparate hydration of adipose and lean tissue require a new model for body water distribution in man. J. Nutr. 106, 1687–1693 (1976).
Google Scholar
Wang, Z. M., Pierson, R. N. Jr. & Heymsfield, S. B. The five-level model: a new approach to organizing body-composition research. Am. J. Clin. Nutr. 56, 19–28 (1992).
Google Scholar
Prado, C. M., Phillips, S. M., Gonzalez, M. C. & Heymsfield, S. B. Muscle matters: the effects of medically induced weight loss on skeletal muscle. Lancet Diabetes Endocrinol. 12, 785–787 (2024).
Google Scholar