Stimulated by Hoheisel et al 2025.[1]
DRG – dorsal root ganglion
FG – fluorogold or fluorochrome yellow
c-fos – a protein from the Fos family widely used as a marker for neuronal activity
c-fos-ir – c-fos immunoreactivity
DH – dorsal horn (of the spinal cord)
VLP – ventral posterolateral nucleus of the thalamus
vlPAG – ventrolateral periaqueductal gray– key to acronyms
I found this paper when looking for references by the same authors for last week’s blog. The name Ulrich Hoheisel is familiar to me from reading basic science papers on muscle pain in the 90’s, and Siegfried Mense is of course a colossus in the field, and someone who has spoken at a BMAS event alongside Bob Gerwin. I seem to recall we referred to them both as ‘the muscle men’.
Talking to Siegfried at dinner prior to the meeting was a real pleasure and intellectually stimulating. It was the time I realised that I was going to learn more from our guest speakers at dinner than I was at the event itself. On a one-to-one basis, researchers can be a little more candid about what they really think is going on. I guess they may feel freer to discuss ideas that are not quite ready for the critical gaze of a more public arena.
One of the references I checked last week was concerning the sizes of DRG cell bodies.[2] It was from the late 80’s, when I qualified, so I was pleasantly surprised to find the two authors appearing again together almost 4 decades later.
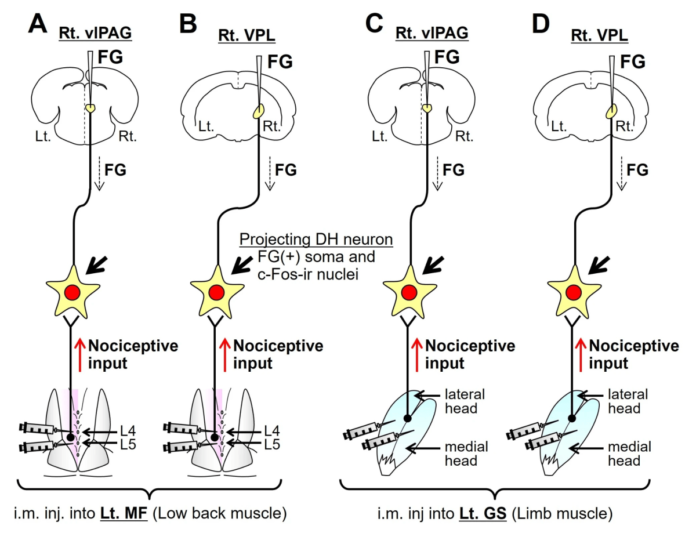
This paper uses retrograde neural tracing with fluorogold (FG) together with c-fos staining following an intramuscular nociceptive stimulus (formalin injection) to compare and contrast the central projections from low lumbar multifidus and gastrocnemius.
The premise of the paper is that the clinical features of chronic muscle pain from the back and the limbs differs and that this may be reflected in the central connections of nociceptive fibres from these different areas.
I was pleased to find a couple of key papers that were not already in my collection related to muscle pain – a review paper in Pain by Mense from 1993 with a whopping 289 references,[3] and a paper on the anatomical sources of back pain by Kellgren from a lecture in 1977,[4] some 40 years after his key papers on muscle pain referral patterns.[5,6]
The diagram above shows 4 different experimental groups combining FG injection in brain nuclei with provocative injections in either multifidus or gastrocnemius. There were actually 8 groups in total because the intramuscular injections were either formalin or saline.
So, what did they find? C-fos-ir in nuclei of DH neurons was positioned laterally for multifidus and more central in the DH for gastrocnemius. This is consistent with the somatotopic distribution of neural input to the DH. The craniocaudal extent of c-fos-ir staining was much greater from multifidus (~6 segments) than from gastrocnemius (~3 segments).
FG labelled neurons from the VPL were all in the deep DH and those from the vlPAG were all in the superficial DH. Double labelled neurons with both FG and c-fos-ir, ie nociceptive neurons that project to these nuclei, covered a greater segmental range from multifidus than from gastrocnemius (6 vs 3 for VPL and 8 vs 6 for vlPAG).
When I saw this greater range of segments involved from multifidus, I was concerned that there may be anatomical differences based on the sites of injection. When needling multifidus there are 3 different segmental muscles in the region, whereas with gastrocnemius is a single muscle albeit with 2 heads (both were injected). I contacted the corresponding author (Toru Taguchi) with my concern, and he kindly sent me one of his papers from 2007, which demonstrated the segments involved when multifidus was injected at L5 with true blue (another retrograde neural marker).[7] Staining was seen over 6 segments with the majority at L2 to L4, peaking at L3. Subsequent formalin injection at L4 and L5 demonstrated more c-fos-ir at L3 than L5.
So, what can we conclude from these anatomical differences in nociception between axial and limb musculature? Well, the good news for those of us using paraspinal segmental approaches is that the spread of segmental influence is greater when targeting multifidus than when using points solely in the limbs.
References
1 Hoheisel U, Treede R-D, Mense S, et al. Central projections of nociceptive input originating from the low back and limb muscle in rats. Sci Rep. 2025;15:2552. doi: 10.1038/s41598-025-86832-z
2 Hoheisel U, Mense S. Observations on the morphology of axons and somata of slowly conducting dorsal root ganglion cells in the cat. Brain Res. 1987;423:269–78. doi: 10.1016/0006-8993(87)90849-3
3 Mense S. Nociception from skeletal muscle in relation to clinical muscle pain. Pain. 1993;54:241–89. doi: 10.1016/0304-3959(93)90027-M
4 Kellgren JH. The anatomical source of back pain. Rheumatol Rehabil. 1977;16:3–12. doi: 10.1093/rheumatology/16.1.3
5 Kellgren JH. Observations on referred pain arising from muscle. Clin Sci. 1938;3:175–90.
6 Kellgren JH. A preliminary account of referred pains arising from muscle. BMJ. 1938;1:325–7. doi: 10.1136/bmj.1.4023.325
7 Taguchi T, John V, Hoheisel U, et al. Neuroanatomical pathway of nociception originating in a low back muscle (multifidus) in the rat. Neurosci Lett. 2007;427:22–7. doi: 10.1016/j.neulet.2007.08.021
Declaration of interests MC
Published