The immunological landscapes of SFMCs and/or PBMCs of PD-1-IA patients, RA patients and HCs
We comprehensively analyzed CD45+ immune cells in the synovial fluid and peripheral blood of patients with IA_act (n = 5) and active seropositive RA (n = 8) using scRNA-seq, flow cytometry, and ELISA. Additionally, CD45+ immune cells were obtained from the paired peripheral blood of IA_rem patients (n = 5) and HCs (n = 8) (Fig. 1a). We also retrieved external scRNA-seq dataset of PBMCs from the patients who received ICI treatment but did not develop irAEs (n = 7) from a published article as additional control11. The median age of PD-1-IA patients at IA onset was 59 years (IQR = 51–62 years), with female predominance (female:male = 4:1). The median disease duration between anti-PD-1 treatment and the onset of arthritis was 97 days (IQR = 70–269 days). Three patients had polyarthritis involving both large and small joints, whilst two patients had oligoarthritis only affecting the bilateral knee joints. The demographics, clinical characteristics, and treatments are listed in the supplementary materials (Supplementary Tables 1, 2).
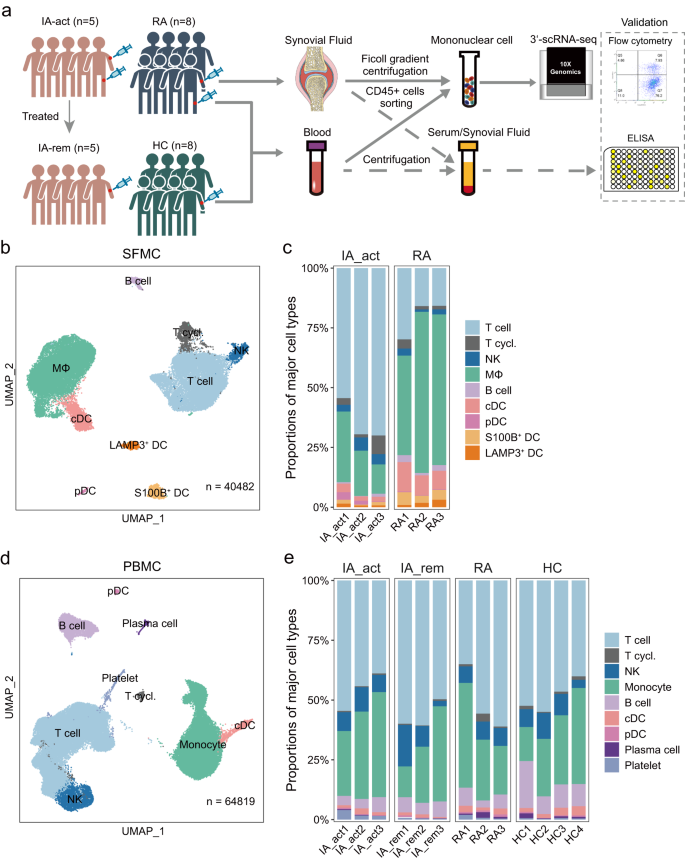
a Workflow showing the collection and processing of samples for scRNA-seq, flow cytometry, and ELISA. b UMAP projection of 9 major cell types in synovial fluid mononuclear cells (SFMCs) (n = 3 individuals per group). c The proportions of major cell types in SFMCs from all individuals in each patient group (IA_act and seropositive RA). d UMAP projection of 9 major cell types in peripheral blood cells (PBMCs) (n = 3–4 individuals per group). e The proportions of major cell types in PBMCs from all individuals in each patient group (IA_act, IA_rem, seropositive RA, and HC). IA_act active inflammatory arthritis, IA_rem inflammatory arthritis in remission, RA rheumatoid arthritis, HC healthy control. MΦ macrophages, T.cycl. cycling T cells.
To investigate the immune cell profile of inflamed joints, we performed scRNA-seq of CD45+ SFMCs in IA patients (n = 3) and seropositive RA patients (n = 3) (Fig. 1a). The combined SFMC scRNA-seq dataset contained 40482 high-quality cells from well-defined immune lineages. To analyze the systemic immunologic responses in PD-1-IA, we performed scRNA-seq of sorted CD45+ PBMCs from IA_act (n = 3) and IA_rem (n = 3) patients and compared the results with data for the same cells from RA patients (n = 3) and HCs (n = 4). The combined PBMC scRNA-seq dataset contained 64,819 high-quality cells from well-defined immune lineages.
Based on unsupervised clustering of SFMCs, 9 major clusters including T cells, cycling T cells, natural killer (NK) cells, macrophages, B cells, conventional dendritic cells (cDCs), plasmacytoid dendritic cells (pDCs), S100B+ dendritic cells (DCs), and LAMP3+ DCs were defined by canonical markers (Fig. 1b, Supplementary Fig. 1a). Striking disparities in SFMCs were observed between IA_act and RA patients. Compared with RA, IA_act showed a significant increase in the proportion of T cells and significant decreases in the proportions of macrophages, cDCs, and S100B DCs in SFMCs (Fig. 1c, d; Supplementary Fig. 1b). The actual counts and flow cytometry of the cell subsets in each sample were shown in Supplementary Fig. 1c, d. Similarly, a total of 9 major cell lineages including T cells, cycling T cells, NK cells, monocytes, B cells, cDCs, pDCs, plasma cells, and platelets were identified by canonical markers in PBMCs (Fig. 1d, Supplementary Fig. 1e). The proportions of major cell lineages, except for that of platelets, did not significantly differ among the four groups (Fig. 1e, Supplementary Fig. 1f). These results showed that IA_act was associated with major changes in T cells and myeloid cells in the synovial fluid but not in the peripheral blood.
Expanded IL1B
hi myeloid cells in PD-1-IA synovial fluid and peripheral blood
To identify PD-1-IA-related cell clusters, we performed unsupervised clustering of myeloid cells. Based on canonical myeloid markers (Supplementary Fig. 2a), 9 subclusters of myeloid cells were defined in SFMCs; these subclusters included 5 monocyte/macrophage subclusters (monocyte-like macrophages, SPP1+ macrophages, IL1Bhi macrophages, CXCL9hi macrophages, and C1QA+ macrophages) and 4 DC subclusters (cDCs, S100B+ DCs, LAMP3+ DCs, and pDCs) (Fig. 2a). The IA_act group was characterized by a predominant proportion of IL1Bhi macrophages, which was almost absent in the RA groups (Fig. 2a–e, Supplementary Figs. 2b, 3a–f). In contrast, the RA groups were significantly enriched in monocyte-like macrophages and SPP1+ macrophages (Fig. 2c). Furthermore, we performed flow cytometry and observed an increased proportion of IL1β+ CD11b+ synovial macrophages from one IA_act patient compared to the one RA patient (Supplementary Fig. 2c, gating strategy in Supplementary Fig. 13a).
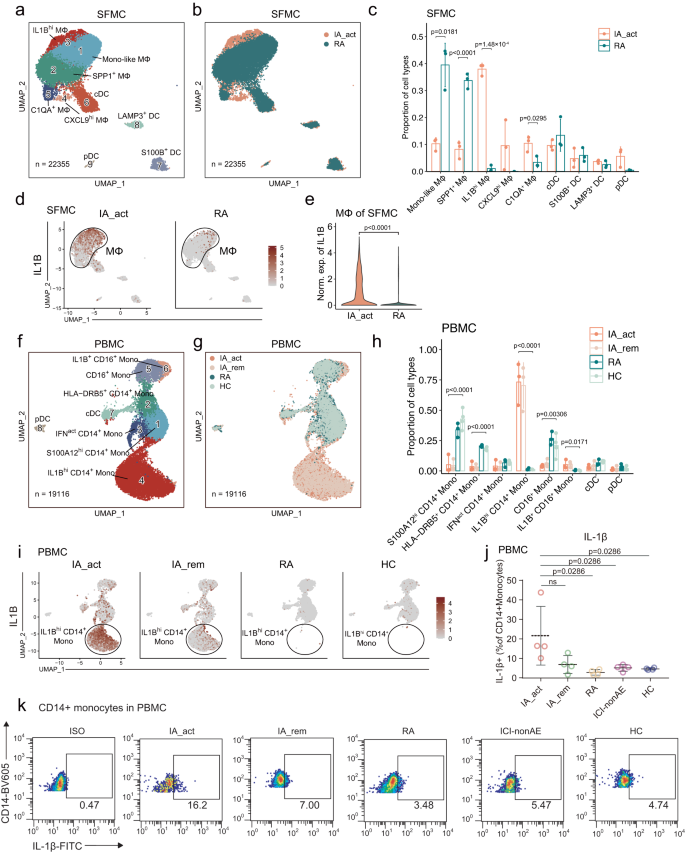
a Identification of 9 subclusters of myeloid cells across all SFMC samples. b Distribution of myeloid cell subclusters of SFMCs between IA_act and RA. c Barplot showing the proportion (mean ± SD) of each myeloid cell subcluster in IA_act and RA with p-values calculated by unpaired two-sided t-tests. d UMAP plots showing IL1B gene expression in myeloid cells in SFMCs from IA_act (left) or RA (right) patients. e Quantification of the differences in IL1B gene expression of macrophages of SFMCs between the IA_act and RA by a two-sided Wilcoxon test. f Identification of 8 subclusters of myeloid cells across all PBMC samples. g Distribution of myeloid cell subclusters in PBMCs among patient groups (IA_act, IA_rem, RA, and HC). h Barplot showing the proportion (mean ± SD) of each myeloid subcluster among the patient groups with one-way two-sided ANOVA tests. i UMAP plots showing the IL1B gene expression of myeloid cells in PBMCs among the patient groups. j Quantification of IL1β+ CD14+ monocytes (percentage of IL1β+ cells in CD14+monocytes, mean ± SD) among the patient groups by flow cytometry. The data show n = 4 biological replicates over three independent experiments. Paired two-sided t-test compared the IA_act group with the IA_rem group. Wilcoxon tests compared the IA_act group with the RA, ICI-nonAE, or HC group. ns, nonsignificant. k Representative flow cytometry plots for (j). ICI-nonAE ICI-treated patients without irAEs, IA_act active inflammatory arthritis, IA_rem inflammatory arthritis in remission, RA rheumatoid arthritis, HC healthy control, ISO isotype, Mono-like monocyte-like, MΦ macrophages.
In PBMCs, 8 subclusters of myeloid cells were defined according to canonical myeloid markers (Supplementary Fig. 2d); these subclusters included 6 monocyte subclusters (S100A12hi CD14+ monocytes, HLA-DRB5+ CD14+ monocytes, IFN-activated [IFNact] CD14+ monocytes, IL1Bhi CD14+ monocytes, CD16+ monocytes, and IL1B+ CD16+ monocytes) and 2 DC subclusters (cDCs and pDCs) (Fig. 2f). Notably, we also observed that the two IL1Bhi subclusters (IL1Bhi CD14+ monocytes and IL1B+ CD16+ monocytes) were nearly exclusive to the IA_act and IA_rem groups (Fig. 2g, h, Supplementary Fig. 2e, Supplementary Fig. 3g–k). Intriguingly, the expression of IL1B was dramatically decreased in the IA_rem group compared with the IA_act group (Fig. 2i). This finding was further validated by flow cytometry (Supplementary Fig.2c, gating strategy in Supplementary Fig. 13a). The fraction of IL1β+ CD14+ monocytes was significantly higher in the IA_act group than in the RA group, ICI-nonAE group and HC group (Fig. 2j, k, gating strategy in Supplementary Fig. 13b). Overall, we found that IL1Bhi myeloid cells were significantly enriched in both the local joint and peripheral blood of PD-1-IA patients, suggesting a pathogenic role for IL1Bhi myeloid cells in PD-1-IA.
IL1B
hi macrophages in SFMCs share similar inflammatory signatures with IL1B
hi monocytes in PBMCs and may differentiate from PBMCs
To identify the molecular pathways and potential treatment targets in PD-1-IA, we investigated the gene expression features of IA-associated myeloid cell subsets: IL1Bhi macrophages in SFMCs and IL1Bhi monocytes in PBMCs. In SFMCs, the top 10 DEGs between IL1Bhi macrophages and other monocyte/macrophage subsets were chemokines (CXCL10, CCL3L1, CCL8), interferon-inducible genes (IFIT2, IRF1, GBP1, GBP5) and genes related to the M1 macrophage phenotype (APOBEC3A12, TNFSF1013, CALHM614) (Fig. 3a). We further performed gene set enrichment analysis (GSEA) with hallmark pathways from MSigDB15. Multiple inflammatory pathways were significantly upregulated in IL1Bhi macrophages and were all designated PD-1-IA-related inflammatory pathways: IFNγ response, IFNα response, TNF signaling through NFκB, IL6-JAK-STAT3 and IL2-STAT5 pathways (Fig. 3b, Supplementary Fig. 4a). Direct comparison of synovial macrophages between the IA_act and RA groups confirmed the upregulation of IA-related inflammatory pathways in IA_act (Supplementary Fig. 4b, 5a, 5b). We investigated the expression of the IA-related pathway at the single-cell level by calculating the AUC scores for each pathway in each cell and further visualized AUC scores with a dimplot. The IL1Bhi macrophage cluster in the SFMCs of IA_act patients significantly upregulated all IA-related inflammatory pathways compared with the other clusters, which indicated the proinflammatory pathogenic roles of IL1Bhi clusters (Fig. 4c, Supplementary Fig. 6a–d).
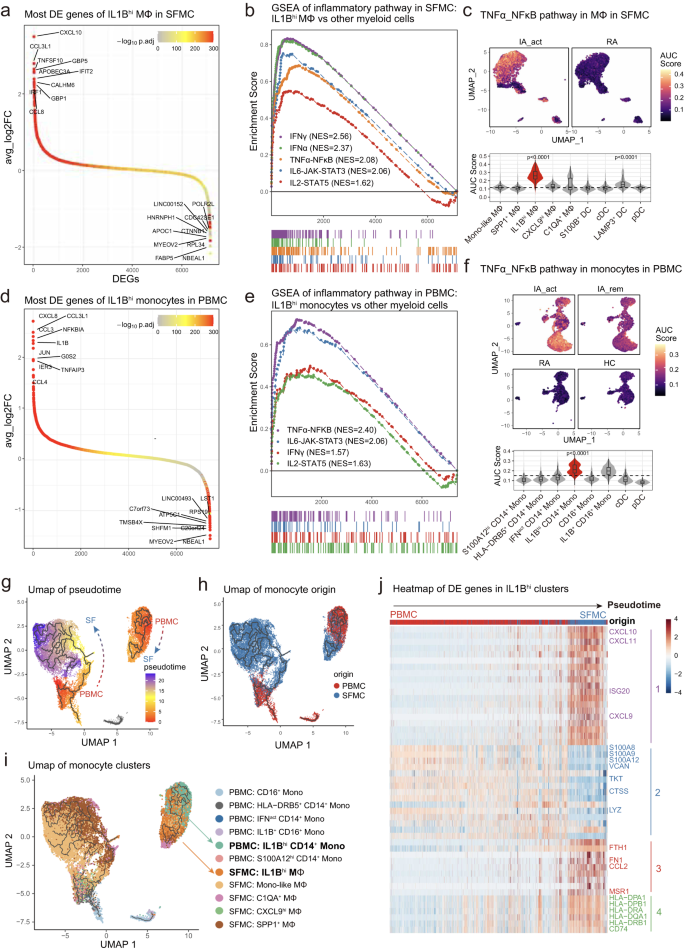
a Differentially expressed genes between IL1Bhi macrophages and other myeloid subclusters in SFMCs, with the top 10 and bottom 10 differentially expressed genes labeled. b Gene set enrichment analysis of the IL1Bhi macrophage subcluster versus other myeloid subclusters in SFMCs for inflammatory pathways. c UMAP visualization (top) and violin and box plot (bottom) showing the TNF_NFκB pathway signature score among myeloid subclusters of SFMCs and between IA_act and RA. The signature score was calculated by the AUCell algorithm (see the “Methods)” section. d Differentially expressed genes between the IL1Bhi monocyte subcluster and other myeloid subclusters in PBMCs, with the top 10 and bottom 10 differentially expressed genes labeled. e Gene set enrichment analysis of IL1Bhi monocytes compared with other myeloid subclusters in PBMCs for inflammatory pathways. f UMAP visualization (top) and violin and box plot (bottom) showing the differences in the TNF_NFκB pathway signature score among myeloid subclusters of PBMCs and patient groups. The signature score was calculated by the AUCell algorithm (see Methods). g Integration of macrophages from SFMCs and monocytes from PBMCs colored by pseudotime. Trajectories are indicated by black lines, developing from low to high pseudotime values. h UMAP distribution of the origin of monocytes/macrophages. i UMAP distribution of subclusters of monocytes/macrophages. The IL1Bhi clusters on the interested trajectory were marked. j Heatmap of the top 50 pseudotime differentially expressed genes of 4 modules across the IL1Bhi CD14+ monocyte-to-IL1Bhi macrophage trajectory. In the box-plots of the bottom panel of (c) (n = 6) and (f) (n = 13), the center lines of the boxes denote the median of the AUC score; the lower and upper limits of the boxes denote the 25% and 75% quantile, respectively. Two-sided Wilcoxon tests comparing the median AUC score of one subcluster with all other clusters. The horizontal dashed line denotes the median AUC score of all the cell clusters, and only the clusters with significantly increased medians were denoted with p-value. IA_act active inflammatory arthritis, IA_rem inflammatory arthritis in remission, RA rheumatoid arthritis, HC healthy control, Mono-like monocyte-like, MΦ macrophages.
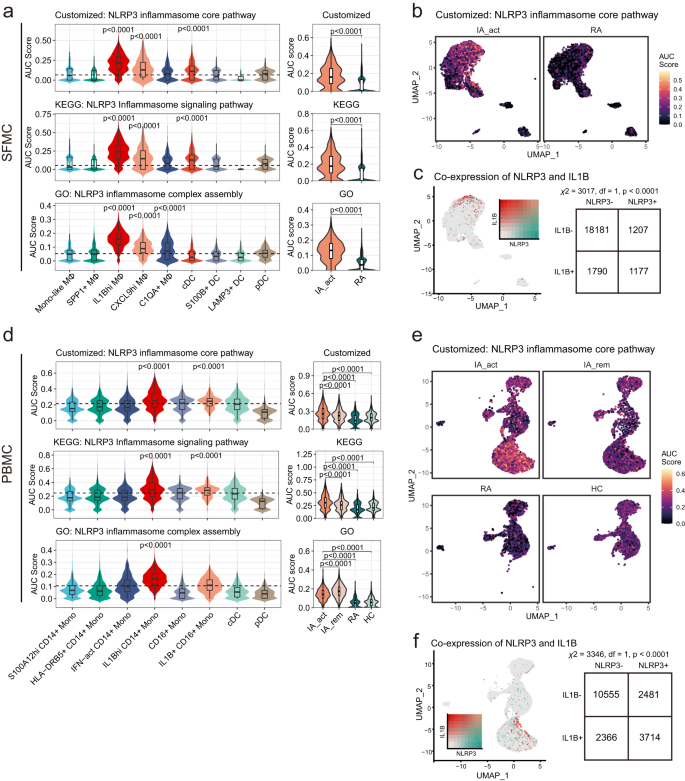
a The differences in NLRP3 inflammasome-related pathway signature scores among subclusters of myeloid SFMCs (left) and between IA_act and RA (right). The AUC was calculated by the AUCell algorithm (see the “Methods” section). b UMAP visualization of customized NLRP3 inflammasome core pathway signature scores across myeloid cells in SFMC in the IA_act and RA groups. c Left, UMAP visualization of the coexpression of the IL1B gene and NLRP3 gene in myeloid SFMCs. Right, a two-sided chi-square test examining the association of NLRP3+ cells with IL1B+ cells in myeloid SFMCs. d The differences in NLRP3 inflammasome-related pathway signature scores among subclusters of myeloid PBMCs (left) and patient groups (right). The AUC was calculated by the AUCell algorithm (see the “Methods” section). e UMAP visualization of the customized NLRP3 inflammasome core pathway signature score across myeloid cells in PBMCs in the IA_act, IA_rem, RA, and HC groups. f Left, UMPA visualization of the coexpression of the IL1B gene and NLRP3 gene in myeloid PBMCs. Right, a two-sided chi-square test examining the association of NLRP3+ cells with IL1B+ cells in myeloid PBMCs. Two-sided Wilcoxon tests comparing one subcluster and all other clusters were applied for the data in (a) and (d). In the box-plots of (a) (n = 6) and (d) (n = 13), the center lines denote the median of the AUC score; the lower and upper limits of the boxes denote the 25% and 75% quantile, respectively. Two-sided Wilcoxon tests comparing the median AUC score of one subcluster with all other clusters. The horizontal dashed line denotes the median AUC score of all the cell clusters, and only the clusters with significantly increased medians were denoted with p-value. IA_act active inflammatory arthritis, IA_rem inflammatory arthritis in remission, RA rheumatoid arthritis, HC healthy control, Mono-like monocyte-like, MΦ macrophages.
Similar to the results for SFMCs, IL1Bhi monocytes in the peripheral blood also demonstrated proinflammatory features. The most significant DEGs of IL1Bhi monocytes in PBMCs were chemokines (CXCL8, CCL3L1, IL1B, CCL4) (Fig. 3d, Supplementary Fig. 7a). GSEA showed that the IA-associated inflammatory pathways were also significantly upregulated in IL1Bhi monocytes and included IFNγ response, TNF signaling through NFκB, IL6-JAK-STAT3 and IL2-STAT5 pathways, among which the TNF signaling pathway ranked above the others (Fig. 3e; Supplementary Figs. 4c, 6e–g, 7b). Further analysis of pathway enrichment also showed that the TNF signaling pathway was the most prominently upregulated pathway in the IA_act group compared with the disease remission, RA and HC groups (Supplementary Fig. 4d–f). Among all the cell clusters, the IL1Bhi CD14+ monocyte cluster exhibited the strongest upregulation of the TNF signaling pathway (Fig. 3f). All IA-associated pathways were significantly upregulated in the IA_act group compared with the RA and HC groups, while inflammatory pathways other than TNF signaling did not differ between active disease and remission (Fig. 3f, Supplementary Fig. 8a–f). Therefore, only the TNF signaling pathway was related to disease activity and possibly the core pathogenic pathway of PD-1-IA. Other upregulated proinflammatory pathways (IFNγ, IFNα, IL6, and IL2) may reflect the systemic immune response induced by ICI administration.
We further analyzed the gene expression kinetics of monocytes and macrophages using the Monocle3 package, an unsupervised algorithm16,17,18. We reconstructed two trajectories from the combined single-cell dataset of monocytes and macrophages from SFMCs and PBMCs (Fig. 3g, h). Notably, IL1Bhi monocytes and macrophages formed a trajectory distinct from other clusters, indicating a close cell transitional order (Fig. 3i). Ordered by pseudotime (an abstract unit of cell transition), the DEGs along the IL1Bhi cell trajectory clustered into 4 distinct modules. Module 1 was mainly composed of proinflammatory genes, while module 4 was mainly composed of marker genes of macrophages. The IL1Bhi cell trajectory reflected the transition from peripheral blood monocytes to synovial fluid proinflammatory macrophages, whose phenotype resembled that of M1 macrophages (Fig. 3j). Based on the genetic dynamics, we assumed that IL1Bhi macrophages may differentiate from peripheral IL1Bhi monocytes.
Elevated NLRP3 inflammasome pathway activity in IL1B
hi myeloid cells from either PBMCs or SFMCs
IL1β is classically produced following the activation of the NLRP3 inflammasome19. To identify the mechanisms of IL1Bhi myeloid cell expansion in PD-1-IA, we examined the NLRP3 inflammasome pathway in IL1Bhi myeloid cells by assessing the signatures of involved genes. In addition to utilizing signatures derived from the GO database and KEGG database15, we generated a customized signature that included only the core genes related to NLRP3 inflammasome activation (NLRP3, PYCARD, CASP1, IL1B, IL18, GSDMD). In SFMCs, the activities of the three signatures were mostly elevated in IL1Bhi macrophages and were significantly enriched in the IA_act group compared with the RA group (Fig. 4a, b; Supplementary Fig. 9a, b). The coexpression of the NLRP3 gene and IL1B gene, as well as the coexpression of NLRP3 inflammasome core pathway components and the IL1B gene, was significant in single-cell transcriptomics (Fig. 4c, Supplementary Fig. 9c).
Similarly, in PBMCs, the activities of NLRP3 inflammasome signatures were mostly elevated in IL1Bhi monocytes and were significantly enriched in the IA group compared with the RA group and HC group (Fig. 4d, e; Supplementary Fig. 9a, b). Furthermore, the activities of NLRP3 inflammasome signatures, except for the NLRP3 inflammasome complex assembly signature, were elevated in the IA_act group compared with the IA_rem group (Fig. 4d). The coexpression of the NLRP3 gene and IL1B gene, as well as the coexpression of NLRP3 inflammasome core pathway components and the IL1B gene, was significant in single-cell transcriptomics (Fig. 4f, Supplementary Fig. 9d). Together, these results indicated the important role of the NLRP3 inflammasome pathway in IL1Bhi myeloid cells and its potential as a therapeutic target in PD-1-IA.
Increase in the CD8+ Tex population in SFMCs of PD-1-IA patients
Next, we examined the synovial T-cell and NK cell compartments in detail. Among the 12 well-defined T/NK subclusters, 5 were CD4+ T cell subclusters (CD4+ central memory T cells [Tcms], SELL+ CD4+ Tcms, CD4+ effector memory T cells [Tems], CD4+ Texs, and CD4+ regulatory T cells [Tregs]), 5 were CD8+ T cell subclusters (CD8+ mucosal-associated invariant T cells [MAITs], CD8+ Tems, CD8+ cytotoxic T cells [CTLs], CD8+ Texs, and cycling CD8+ T cells), and 2 were NK cell subclusters (FCGR3A+ NK cells and XCL2+ NK cells) (Fig. 5a, b). Compared with the RA groups, the IA_act groups displayed significantly higher fractions of CD8+ Texs, CD8+ Tems and CD4+ Tregs but significantly lower fractions of CD4+ Tems and CD4+ Texs (Fig. 5c, Supplementary Fig. 10). The identity of each T/NK cell subcluster was labeled based on marker genes of the T/NK cell lineage and functions (Supplementary Fig. 11a, b). To comprehensively understand the differences in T cells between IA_act and RA, we evaluated the expression of panels of genes in 4 categories, namely, exhaustion markers (PDCD1, CTLA4), inflammation markers (IFNG, CXCL13), trafficking markers (CXCR3, CXCR6) and cytotoxicity markers (GZMB, PRF1), in subclusters of interest (Fig. 5d, e; Supplementary Fig. 10c). Generally, the expression of these markers was significantly higher in T cells in IA_act than those in RA, suggesting more functional states of T cells were present in IA_act than in RA. An exception was observed in CD8+ Tems, which were significantly enriched in IA_act but exhibited insignificant differences in the expression of these markers between IA_act and RA.
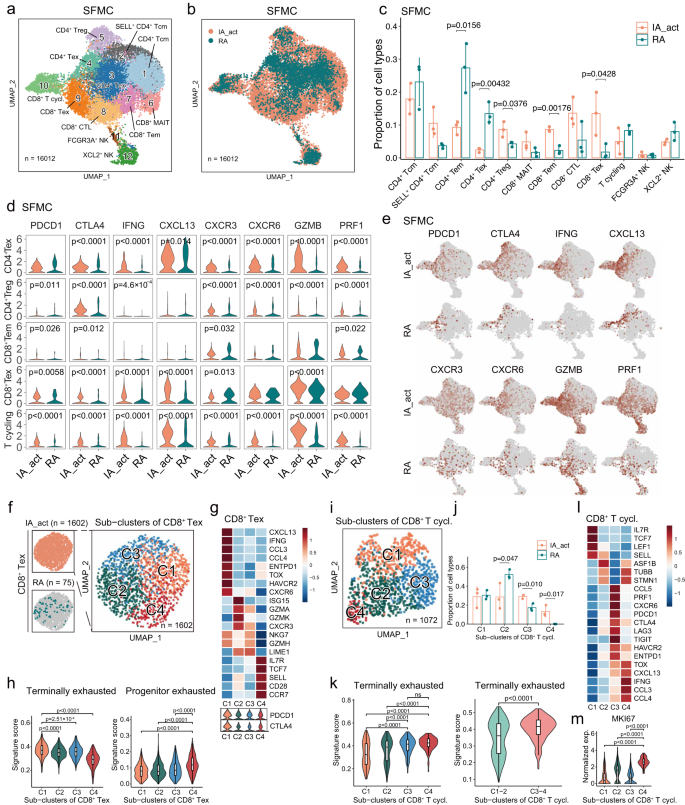
a Identification of 12 subclusters of T and NK cells across all SFMC samples. b Distribution of T/NK cell subclusters of SFMCs between IA_act and RA. c Quantification of the fraction (mean ± SD) of each T/NK subcluster between the patient groups with a two-sided t-test. d Violin plots showing the expression of genes of interest in selected T/NK cell subclusters between IA_act and RA with a two-sided Wilcoxon test. e UMAP plots showing the expression of genes of interest in T/NK cell subclusters between IA_act and RA. f Subclustering of 1602 exhausted CD8+ T cells from the SFMCs of IA_act patients into 4 subsets. g Heatmap showing the expression of marker genes across subsets of exhausted CD8+ T cells (CD8+ Texs) (top) and violin plot showing the expression of the PDCD1 and CTLA4 genes across subsets of CD8+ Texs (bottom). h Quantification of terminally exhausted (left) and progenitor exhausted signature scores (right) among CD8+ Texs with an unpaired two-sided Wilcoxon test. Signature scores were calculated by the AUCell algorithm (see the “Methods” section). i UMAP plot showing 1072 cycling CD8+ T cells in SFMCs from all individuals separated into 4 subsets. j Quantification of the subcluster proportions (mean ± SD) between IA_act and RA with a two-sided t-test. k Quantification of the differences in the terminally exhausted signature score among 4 subsets of cycling CD8+ T cells (left) and between the C1-2 subset and C3-4 subset (right) with a two-sided Wilcoxon test. l Heatmap showing the expression of marker genes across subsets of cycling CD8+ T cells. m. Quantification of the MKI67 gene expression between the C4 subset and other subsets with a two-sided Wilcoxon test. In the box-plots of (h), (k), and (m), the center lines denote the median of the signature score or normalized gene expression in each subset; the lower and upper limits of the boxes denote the 25% and 75% quantile, respectively. IA_act active inflammatory arthritis, IA_rem inflammatory arthritis in remission, RA rheumatoid arthritis, HC healthy control, T.cycl. cycling T cells.
Given that CD8+ Texs were nearly exclusive to SFMCs of IA_act patients, we extracted the SFMC-derived CD8+ Texs in the IA_act group and further subgrouped them into 4 clusters (C1– C4) to explore potential heterogeneity (Fig. 5f). Intriguingly, CD8+ Tex-C1 was marked by elevated expression of terminal exhaustion markers (PDCD1, ENTPD1, HAVCR2, TOX), whereas CD8+ Tex-C4 was marked by elevated expression of progenitor exhausted cell markers (PDCD1, TCF7, IL7R, SELL) (Fig. 5g). In cancer immunotherapy models, CD8+ T cells have been reported to include a subset containing a progenitor exhausted population, which includes cells that are self-renewing, promote tumor control induced by ICIs, and eventually differentiate into a terminally exhausted population20. To validate whether CD8+ Tex-C4 exhibits the progenitor-exhausted phenotype, we scored each cell with well-established terminally and progenitor-exhausted gene signatures (Supplementary Table 3). The results revealed that CD8+ Tex-C4 exhibited significantly higher activity for the progenitor exhausted signature. Conversely, CD8+ Tex-C1 was significantly enriched in the terminally exhausted signature (Fig. 5h).
Although the cell fraction of cycling CD8+ T cells did not differ between the IA_act and RA groups, we identified distinct exhaustion statuses for cycling CD8+ T cells between the two groups, suggesting the existence of exhausted subpopulations related to IA_act (Fig. 5d). Therefore, we performed graph-based subclustering of cycling CD8+ T cells and obtained 4 distinct clusters (C1–C4) (Fig. 5i). Notably, CD8+ T cycling-C3 and CD8+ T cycling-C4, which were associated with IA_act (Fig. 5j), displayed a significantly elevated activity score for the terminally exhausted signature (Fig. 5k). Mirroring CD8+ Tex-C1, CD8+ T cycling-C4 was characterized by high transcriptomic expression of CXCL13, TOX, IFNG, CCL3, and CCL4 (Fig. 5l) and exhibited a much stronger proliferative capacity indicated by the expression of MKI67 (Fig. 5m). Together, these observations indicated that self-renewing progenitor exhausted CD8+ Texs (CD8+ Tex-C4) and proliferative CD8+ Texs (CD8+ T cycling-C4) could both contribute to the pathogenesis and especially the persistence of PD-1-IA.
IL1B
hi myeloid cells orchestrate cell communication through the CCR1-CCL5/CCL3 and CXCL10-CXCR3 axes
To further elucidate the crosstalk between the expanded IL1Bhi myeloid cell and T cell clusters in PD-1-IA, we utilized CellPhoneDB21, a ligand‒receptor interaction database, to identify cytokine–receptor and chemokine–receptor interaction pairs in both the myeloid cell and T-cell populations. In the synovial fluid, myeloid cells in the PD-1-IA group displayed much stronger interactions with T cells than did those in the RA group (Fig. 6a). Generally, in the peripheral blood, the interaction pairs of cytokines/chemokines and receptors in the PD-1-IA group outnumbered those in the RA and HC groups, and the number was slightly higher in the IA_act group than in the IA_rem group (Fig. 6b). In both the peripheral blood and synovial fluid, the IL1Bhi clusters exhibited strong interactions with multiple T-cell subsets including CD4+ and CD8+ Tems, CD4+ and CD8+ Texs and cycling CD8+ T cells. Overall, in the PD-1-IA group, IL1Bhi myeloid cells centered the immune response through interactions with T cells, both systemically and locally at inflamed joints.
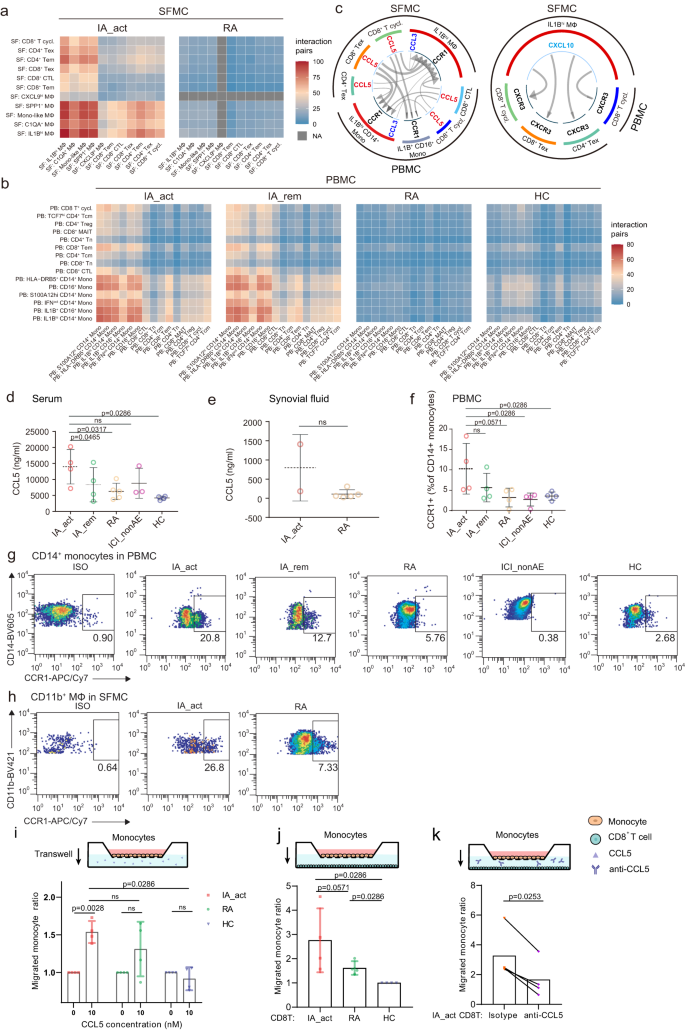
a and b Number of interaction pairs between monocytes and T cells in PBMCs (a) and in SFMCs (b) in each patient group. All the interactions were statistically significant by permutation tests. c Significant chemokine ligand‒receptor pairs across IL1Bhi myeloid cells and T cells in PBMCs and SFMCs. CCL5-CCR1 and CCL3-CCR1 (left) and CXCL10-CXCR3 (right) ligand‒receptor pairs are presented separately. The direction of the arrows indicates the interaction between the ligand to the receptor. d and e Quantification of the serum (d) and synovial fluid CCL5 (e) protein concentration (mean ± SD) among patient groups. The data show n = 2–5 biological replicates over two independent experiments. f Flow cytometry showing the percentages (mean ± SD) of CCR1+ CD14+ monocytes in PBMCs among patient groups (n = 4 per group, gating strategy in Supplementary Fig. 13b). The data show n = 4 biological replicates over three independent experiments. Paired two-sided t-test compared the IA_act with IA_rem in (d) and (f). Two-sided Wilcoxon tests compared the IA_act with the RA, ICI-nonAE, or HC in (d–f). g Representative flow cytometry plots for (f). h Flow cytometry showing the percentages of CCR1+ macrophages in SFMCs in IA_act and RA patients (gating strategy in Supplementary Fig. 13a). i Transwell migration of monocytes under the CCL5 treatment by the chemotaxis assay. The migrated cell ratio was the division of migrated cell counts with CCL5 treatment to those without CCL5 treatment, comparing with ratio paired-t tests within the patient groups, and unpaired two-sided Wilcoxon tests among the patient groups (IA_act, HC, and RA). j Transwell migration of monocytes towards T cells among the patient groups (IA_act, HC, and RA), comparing with unpaired two-sided Wilcoxon tests. k Transwell migration of monocytes towards T cells in IA_act, with or without CCL5 blockade, comparing with a two-sided ratio paired t-test. The data show n = 4 biological replicates over three independent experiments. ns nonsignificant, Mono-like monocyte-like, IA_act active inflammatory arthritis, IA_rem inflammatory arthritis in remission, RA rheumatoid arthritis, HC healthy control, MΦ macrophages, Mono monocytes, T.cycl. cycling T cells.
We further investigated the cytokine/chemokine–receptor pairs employed by IL1Bhi myeloid cells. The top 50 interaction pairs in PD-1-IA-associated clusters were filtered and plotted (Supplementary Fig. 12a). The dominant cytokine/chemokine–receptor pairs were CCR1-CCL5/CCL3 and CXCL10-CXCR3 (Fig. 6c). We validated the expression of CCR1-CCL5/CCL3 and CXCL10-CXCR3 in the IA_act group and further compared it with that in the IA_rem, RA, ICI-nonAE, and HC groups through ELISA and flow cytometry. The median synovial fluid level of CCL5 was higher in patients with IA_act than in RA patients, although the difference was not statistically significant due to the limited sample size (Fig. 6e). Notably, the serum level of CCL5 in patients with IA_act was significantly higher than that in patients with IA_rem or that in controls (Fig. 6d). Similarly, the percentages of both CCL3+ CD14+ peripheral monocytes and CCL3+ CD11b+ synovial macrophages in IA_act exceeded those in RA (Supplementary Fig. 12b–d, gating strategy in Supplementary Fig. 13a, b). As the receptor for CCL5 and CCL3, CCR1 also had higher expression in synovial CD11b+ macrophages and peripheral CD14+ monocytes in IA_act compared with those in RA and controls (Fig. 6f–h, gating strategy in Supplementary Fig. 13a, b). The ex-vivo chemotaxis assay validated that the peripheral monocytes isolated from IA_act patients showed increased transwell migration under the CCL5 treatment (Fig. 6i). Additionally, increased migration of the monocytes towards the CD8+ T cells was observed in IA_act patients, and the enhanced migration was attenuated through the CCL5 blockade (Fig. 6j, k). Consistently, for the CXCL10-CXCR3 interaction pair, flow cytometry indicated that CXCL10+ CD11b+ macrophages were also expanded in the synovial fluid, and CXCR3+ T cells accumulated in the peripheral blood of IA_act patients (Supplementary Fig. 12e–h, gating strategy in Supplementary Fig. 13a, c). In the chemotaxis assay, CD8+ T cells from IA_act showed significantly enhanced migration with CXCL10 treatment (Supplementary Fig. 12i). Overall, we demonstrated that CCR1-CCL5/CCL3 and CXCL10-CXCR3 were key signaling pairs between IL1Bhi myeloid cells and CD8+ Texs in PD-1-IA.


