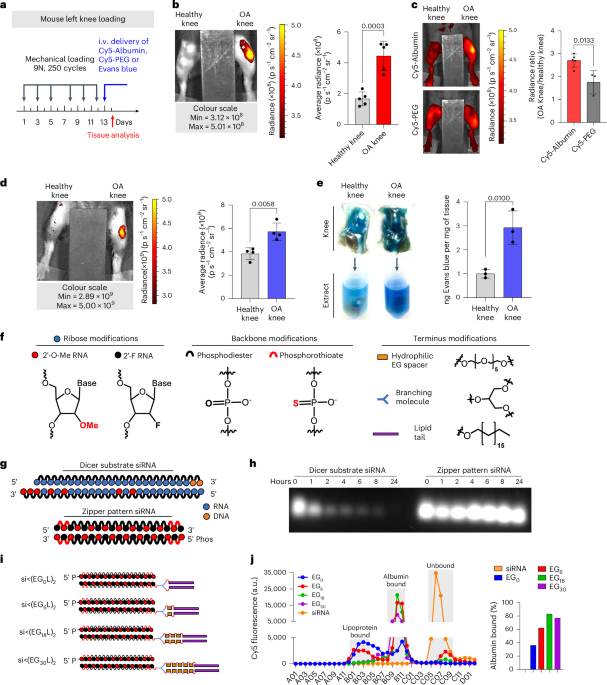
Circulating albumin robustly accumulates in PTOA joints
To determine the extent to which circulating albumin accumulates in arthritic joints, we intravenously (i.v.) delivered Cy5-labelled mouse serum albumin (MSA-Cy5) into mice with induced PTOA. In this model, the left knee undergoes repeated loading to create damage and inflammation, while the right knee remains unchallenged (Fig. 1a). At 24 h after delivery, abundant MSA-Cy5 was observed in the synovium and cartilage of PTOA knees but not in contralateral control knees (Fig. 1b and Supplementary Fig. 1). In contrast, Cy5-conjugated poly(ethylene glycol) (PEG-Cy5) did not accumulate preferentially in PTOA knees (Fig. 1c). Further, i.v. delivery of the albumin binding dye Evans blue resulted in preferential accumulation in synovia and cartilage of PTOA knees, but not contralateral healthy knees (Fig. 1d,e, and Supplementary Figs. 2 and 3). Interestingly, mRNAs of genes encoding the albumin delivery/transport factors caveolin-1 and secreted protein acidic and rich in cysteine (SPARC) were elevated in PTOA joint tissues (Supplementary Fig. 3d), consistent with previous reports of SPARC upregulation in arthritic joints53,54,55,56. Further, interstitial SPARC expression in tumours correlates with efficacy of albumin-based therapeutics in head and neck cancers57. These findings support the idea that hitchhiking on endogenous circulating albumin might be used to skew the biodistribution of siRNA conjugates to arthritic joints following i.v. delivery.
a–d, Left-knee mechanical loading was used to induce unilateral PTOA in mice, followed by i.v. delivery of 3 mg kg−1 MSA-Cy5 (N = 5), 3 mg kg−1 PEG-Cy5 (N = 3) and 200 µl of 2% w/v Evans blue (N = 4). Cy5 and Evans blue were measured by fluorescence imaging 24 h after delivery. Representative images are shown. a, Experimental timeline. b, MSA-Cy5 fluorescence in paired PTOA and healthy knees. c, Ratio of MSA-Cy5 and PEG-Cy5 fluorescence in PTOA and healthy knees for each treated mouse. d, Intravital Evans blue fluorescence in paired contralateral PTOA and contralateral non-loaded knees. e, PTOA and contralateral non-loaded knees were excised 24 h after Evans blue delivery. Evans blue was extracted and measured by densitometry (N = 3). Representative images of joints and resulting joint extracts are shown. f, Chemical modifications of siRNA ribose, backbone and terminus. g, Schematic of synthetic dicer-substrate siRNA and alternating 2′F and 2′OMe modified ‘zipper’ siRNA. h, siRNA stability in OA-derived synovial fluid: representative gel electrophoresis of dicer substrate and zipper siRNA sequences after incubation in synovial fluid collected from an untreated OA patient. i, Schematic representation of the si<(EGXL)2 series of zipper-modified siRNAs with divalent lipid end-modifications with variations in EG content. j, Left: FPLC chromatograph of elution of si<(EGXL)2 variants pre-incubated with human synovial fluid form normal joints. Right: percentage of total si<(EGXL)2 bound to albumin fractions. All error bars indicate s.d. of biological replicates. Knees labelled healthy indicate a non-loaded contralateral limb (right).
Source data
Enhanced stability, albumin binding and PTOA joint accumulation of optimized siRNA–lipid conjugates
Carrier-free siRNA conjugates must be chemically modified to protect against degradation by endo- and exonucleases. To stabilize the siRNAs, we engineered blunt-ended 19-mer RNA strands with alternating 2′-O-Me and 2′-F ribose modifications in a ‘zipper’ pattern and replaced phosphodiesters with phosphorothioate (PS) linkages for the last two bases of the 5′- and 3′-termini of the sense and antisense strands (Fig. 1f,g). Stability of the zipper-modified siRNA was assessed in synovial fluid from a treatment-naive OA patient, revealing siRNA stability through at least 24 h at 37 °C, whereas a more lightly modified dicer-substrate siRNA was >50% degraded within 1 h (Fig. 1h). Given the stability of the zipper-modified siRNA, all further siRNA modifications were done using this modification pattern.
The zipper siRNAs were end modified with bivalent C18 lipids intended to promote binding of the siRNA into the natural fatty acid (FA) binding pockets of albumin (Fig. 1i)32. A library of bivalent lipid structures was synthesized as recently reported51. Briefly, a splitter phosphoramidite (<) was added at the 5′ end of the sense strand, followed by addition of 0 (EG0), 1 (EG6), 3 (EG18) or 5 (EG30) hexa-ethylene glycol (EG6) phosphoramidite spacers to each branch. Both branches were terminally appended with a C18 lipid, thus generating siRNA<(EGxL)2, where X is the number of EG repeats linking the splitter to each terminal C18.
Binding of the different siRNA structures to albumin within human synovial fluid was assessed using fast protein liquid chromatography (FPLC). A proportion of each of the siRNA<(EGXL)2 conjugates was found in the albumin-containing synovial fluid fractions (Fig. 1j), whereas unconjugated, zipper-pattern modified siRNA was not. Notably, >80% of the total siRNA<(EG18L)2 detected by FPLC was found in the albumin-containing fraction, higher than any other siRNA–lipid conjugate tested. This observation was consistent with the previously measured high affinity binding of siRNA<(EG18L)2 to albumin (KD = 30 nM)51.
Cy5-labelled siRNA<(EGXL)2 constructs were next delivered i.v. (1 mg kg−1) to mice with PTOA induced in the left knee (Fig. 2a). While each accumulated to a greater extent in PTOA knees over healthy contralateral knees, siRNA<(EG18L)2 achieved the highest accumulation in PTOA joints (Fig. 2b and Supplementary Fig. 4). A benchmarking study was done to compare siRNA<(EG18L)2 to commonly used cholesterol-conjugated siRNA (siRNA-Chol). In contrast to the >4-fold enrichment of siRNA<(EG18L)2 in PTOA knees over healthy knees, parental siRNA and cholesterol-conjugated siRNA-Chol each showed only a 1.5- and 2-fold enrichment of delivery to PTOA vs healthy knees, respectively (Fig. 2c). Histological cryosections of PTOA knee synovium and cartilage illustrated holistic joint tissue penetration and cellular uptake of Cy5-labelled siRNA<(EG18L)2 (Supplementary Figs. 5–7), while siRNA-Chol was observed but at lower magnitude within PTOA joint compartments (Fig. 2d). Organ biodistribution studies performed by ex vivo Cy5 imaging showed that liver was the main off-target delivery site for both siRNA<(EG18L)2 and siRNA-Chol, while unconjugated siRNA accumulated primarily in kidneys (Supplementary Fig. 8), a major site of siRNA clearance.
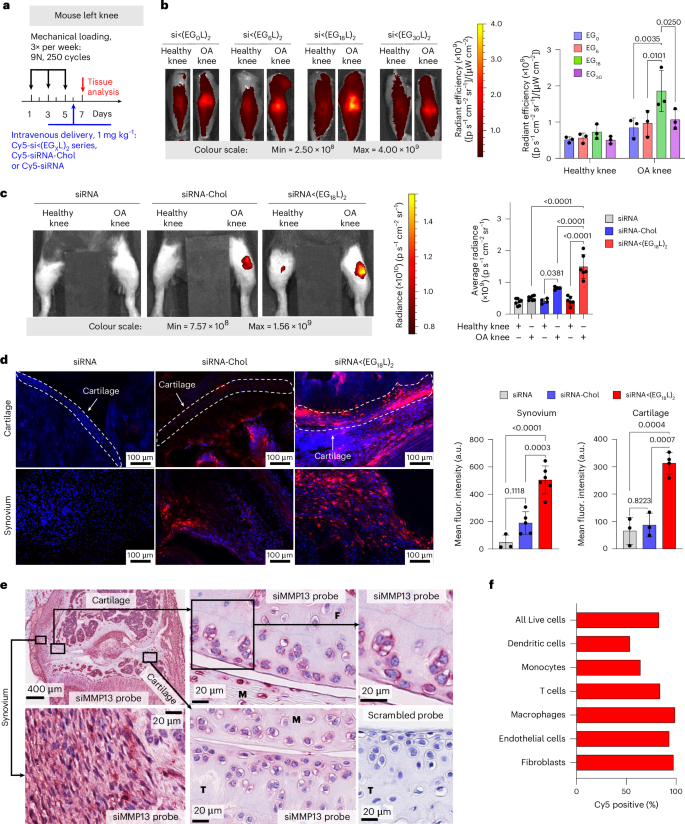
a, Unilateral left knee mechanical loading, treatment (1 mg kg−1 i.v.) and endpoint protocol used in b–d. b, Representative ex-vivo IVIS images and quantitation of Cy5-labelled siRNA<(EGxL)2 accumulation in mouse knees 24 h after delivery. c, Left: representative intravital Cy5 fluorescence images taken 24 h after delivery of Cy5-labelled siRNA, siRNA-Chol or siRNA<(EG18L)2. Right: quantification of average Cy5 fluorescence in knees taken 24 h after mouse i.v. injection. Analysed using mixed effects analysis. d, DAPI-counterstained cryosections of knees from treated mice imaged and quantified for Cy5-labelled siRNA fluorescence in cartilage and synovial tissue. Dashed lines outline articular cartilage. e, In situ hybridization with an siMMP13 probe showing signal localization of siMMP13<(EG18L)2 in mice treated i.v. with 10 mg kg−1. Representative images show siRNA presence in the femoral cartilage (F), meniscus (M), tibial cartilage (T) and synovium. Serial tissue sections incubated with a negative control scrambled probe showed no signal. f, Flow cytometry assessment of cell type-specific uptake of Cy5-siRNA<(EG18L)2 in synovium. Mice were treated i.v. with 10 mg kg−1 following three bilateral mechanical loading sessions over a week. Cells isolated from synovia of 5 mice were pooled for analysis. Experimental and flow cytometry gating details are in Supplementary Fig. 11. All error bars indicate s.d. of biological replicates. Knees labelled healthy indicate a non-loaded contralateral limb (right).
Source data
Interactions between albumin and siRNA<(EG18L)2 were further assessed by FPLC of synovial fluid from OA, RA or normal joints. Similar to what was observed using healthy patient-derived synovial fluid, Cy5-siRNA<(EG18L)2 eluted primarily in the albumin-containing fractions of OA patient and RA patient-derived synovial fluid samples, but with substantially greater intensity in arthritic samples (Supplementary Fig. 9a). In contrast, the parent Cy5-siRNA remained predominantly unbound. Western blotting confirmed the increased albumin content in OA- and RA-derived synovial fractions B12 and C1, the same fractions that siRNA<(EG18L)2 was predominantly associated with (Supplementary Fig. 9b). Complementary native gel electrophoresis analyses of synovial fluid fractions B12-C1 revealed co-localization of Cy5-siRNA<(EG18L)2 with a Coomassie-stained protein of 66 kDa, the known molecular weight of albumin (Supplementary Fig. 9c). Further, siRNA<(EG18L)2 showed stability in synovial fluid from OA joints for at least 96 h (Supplementary Fig. 9d). Taken together, these findings allowed us to assign siRNA<(EG18L)2 as our lead siRNA–lipid conjugate for albumin hitchhiking to arthritic joints, motivating a focus on this construct for subsequent therapeutic studies. We also found that siRNA<(EG18L)2 interacted with albumin across multiple species, including human, mouse and guinea pig, supporting its utility for all the OA and RA models used herein (Supplementary Fig. 9e).
PTOA joint accumulation and cell type-specific uptake of siRNA<(EG18L)2
RNA in situ hybridization (ISH) was utilized for label-free visualization of tissue localization and penetration of the siMMP13<(EG18L)2 delivered i.v. at a dose of 10 mg kg−1. Application of an ISH probe designed against siMMP13 to PTOA joint histological sections revealed the presence of siMMP13<(EG18L)2 within the meniscus and femoral/tibial cartilage, in addition to synovial localization (Fig. 2e). This label-free method illustrates that the therapeutically active siMMP13<(EG18L)2 compound achieves delivery and penetration into important joint tissues with known relevance to PTOA progression. To validate the reproducibility and specificity of the ISH approach, we also observed positive staining with a probe designed against the endogenous small nuclear RNA RNU6; furthermore, application of a negative control probe to the histological sections taken from PTOA joints of mice treated with siMMP13<(EG18L)2 or a saline vehicle control showed no visible staining, as did application of the siMMP13 probe to PTOA joint sections from saline-treated mice (Fig. 2e and Supplementary Fig. 10).
To characterize cell type-specific uptake of the selected siRNA<(EG18L)2 conjugate, Cy5-siRNA<(EG18L)2 (10 mg kg−1) or vehicle was delivered i.v. to mice with mechanical load-induced PTOA in both knees. Cell type-specific uptake by synovial fibroblasts, endothelial cells, macrophages, monocytes, dendritic cells and T cells was characterized 24 h after treatment using flow cytometry (Fig. 2f and Supplementary Fig. 11). A high percentage (83.1%) of the overall cells in the synovium were positive for uptake of Cy5-labelled siRNA<(EG18L)2, with fibroblasts, macrophages and endothelial cells exhibiting the highest proportion of Cy5-positive cells (>90%).
siRNA<(EG18L)2 accumulation in PTOA joints following subcutaneous and intra-articular delivery
Subcutaneous injection of siRNA<(EG18L)2 was tested as an alternative to the i.v. delivery route, given the utility of subcutaneous injections for patient self-administration of biologic drugs. Subcutaneous delivery of Cy5-siRNA<(EG18L)2 (2 mg kg−1) enabled preferential siRNA accumulation in PTOA knees over contralateral non-injured knees, with similar organ biodistribution profile as i.v. delivery (Extended Data Fig. 1a–c). However, subcutaneous delivery of siRNA<(EG18L)2 at 2 mg kg−1 did not achieve the absolute level of siRNA accumulation in PTOA knees seen with i.v. delivery at 1 mg kg−1 (Extended Data Fig. 1d). Notably, a large amount of the Cy5-siRNA<(EG18L)2 dose was retained at the injection site (data not shown), perhaps due to its rapid interaction with subcutaneous fat or cells at the injection site58. Intra-articular (i.a.) delivery, a common clinical route for steroid delivery, was also tested (0.25 mg kg−1), revealing greater Cy5-siRNA<(EG18L)2 retention in the PTOA over healthy knee and greater retention than Cy5-siRNA at 48 h post injection, achieving penetration into cartilage and synovia (Extended Data Fig. 1e–g and Supplementary Fig. 7b). While i.a.-delivered Cy5-siRNA<(EG18L)2 was retained primarily in the knee, Cy5-siRNA redistributed to kidneys, reflecting its relatively rapid removal from the joint by synovial drainage via vasculature and lymphatics, underscoring the importance of the lipid moieties for retention of siRNA<(EG18L)2 within the joint space.
Potent MMP13 knockdown in PTOA-afflicted joints by siRNA<(EG18L)2
siRNA sequences against mouse and guinea pig MMP13 (siMMP13) were screened in mouse ATDC5 chondrogenic cells and primary guinea pig chondrocytes, respectively, for MMP13 knockdown potency (Supplementary Fig. 12). Lead candidate sequences were then synthesized with the zipper modification pattern and MMP13 silencing potency reconfirmed upon transfection into TNF-stimulated ATDC5 cells (Supplementary Fig. 13). Albumin-binding zipper-pattern siMMP13 was then synthesized, thus generating siMMP13<(EG18L)2. Importantly, siMMP13<(EG18L)2, but not the parent zipper-pattern modified siMMP13, provided robust carrier-free (no lipofection reagent) Mmp13 knockdown (Supplementary Fig. 13). The siRNA<(EG18L)2 structure also showed efficient cellular uptake in ATDC5 cells; level of uptake was partially diminished in the presence of excess albumin but increased upon treatment with pro-inflammatory TNF stimulation, even in the presence of excess albumin (Supplementary Fig. 14).
MMP13 silencing by siMMP13<(EG18L)2 in PTOA-affected mouse knees was first assessed 5 days after i.v. treatment, finding >50% Mmp13 knockdown with a 5 mg kg−1 dose and >60% with a 10 mg kg−1 dose (Extended Data Fig. 2a). Neither unconjugated siMMP13 nor siMMP13-Chol (each at 10 mg kg−1 i.v.) significantly affected Mmp13 levels in PTOA knees. A single i.a. injection of siMMP13<(EG18L)2 at 1 mg kg−1 also decreased Mmp13 in PTOA knees (Extended Data Fig. 2b). While subcutaneous delivery of siMMP13<(EG18L)2 at a relatively high dose (50 mg kg−1) diminished Mmp13 in PTOA knees (Extended Data Fig. 2c), a lower dose (20 mg kg−1) reduced Mmp13 in PTOA knees only when co-delivered with an excess of mouse albumin (1:5 molar ratio). These results show promise for use of siMMP13<(EG18L)2 with multiple delivery routes. However, subcutaneous delivery may benefit from more optimization of excipients or penetration enhancers to improve absorption from the injection site into the systemic circulation.
Immunohistochemical (IHC) analysis of PTOA knees revealed abundant MMP13 in articular cartilage, synovium and meniscus compared with tissues in healthy knees. PTOA-induced MMP13 protein signal was markedly reduced in each PTOA knee compartment at 5 days after treatment with siMMP13<(EG18L)2 delivered at either 5 or 10 mg kg−1. However, neither siMMP13-Chol nor siMMP13 impacted MMP13 levels in PTOA knees (Extended Data Fig. 2d). MMP13 protein was undetectable in liver or kidneys by IHC (Extended Data Fig. 2e). RNA expression analyses similarly did not detect Mmp13 in liver, although low levels were found in kidney, albeit 6,000-fold lower than that detected in PTOA knees (Extended Data Fig. 2f). Regardless, kidney Mmp13 gene expression was unaffected in siMMP13<(EG18L)2-treated mice (Extended Data Fig. 2g), supporting the idea that siMMP13<(EG18L)2 might have a wide therapeutic window, with minimal risk of molecularly on-target side effects in two of the primary organs commonly associated with siRNA clearance, liver and kidney.
To further characterize cellular sources of MMP13 in PTOA synovium, we analysed single-cell RNA-sequencing from a murine anterior cruciate ligament (ACL) rupture-induced PTOA model59. Synovial fibroblasts, specifically Thy1+ Pdgfra+ Prg4− sublining fibroblasts were the primary Mmp13-expressing cell type in synovium, with some myeloid cells exhibiting a much lower degree of Mmp13 expression. While healthy synovium was essentially devoid of Mmp13-expressing cells, PTOA synovium exhibited a large increase in the number of Mmp13-positive synovial fibroblasts (Supplementary Fig. 15). Taken together with Cy5-siRNA<(EG18L)2 uptake results demonstrating a high degree of uptake by synovial fibroblasts (Fig. 2f and Supplementary Fig. 11), these results suggest that our siRNA<(EG18L)2 structure efficiently targets the primary Mmp13-expressing cells in synovium.
Sustained retention of siMMP13<(EG18L)2 in PTOA joints enables potent and durable MMP13 knockdown
Mice were subjected to mechanical left knee loading (3 times per week) over 5 weeks. They were i.v. treated with a single dose of Cy5-siRNA<(EG18L)2 at 10 mg kg−1 after the first week of loading, and longitudinal measurements of Cy5 retention in PTOA knees were performed at time points throughout the next 30 days (Extended Data Fig. 3a). At day 1, there was significant Cy5 signal in the cartilage/meniscus and synovial tissues (Extended Data Fig. 3b) that was retained for up to 30 days in PTOA knees treated systemically with Cy5-siRNA<(EG18L)2 (Extended Data Fig. 3c), and higher siRNA<(EG18L)2 levels were detected in PTOA knees over contralateral control knees (Extended Data Fig. 3d,e). Importantly, a single siMMP13<(EG18L)2 treatment maintained knockdown of MMP13 transcript and protein in PTOA joints through day 30 (Extended Data Fig. 3f,g).
Intra-articular dosing of siRNA<(EG18L)2 (1 mg kg−1) was similarly assessed, revealing preferential retention of Cy5-siRNA<(EG18L)2 in PTOA knees over contralateral healthy knees through 30 days post treatment (Extended Data Fig. 4). In a side-by-side comparison, an i.v. dose of 10 mg kg−1 and intra-articular dose of 1 mg kg−1 siRNA<(EG18L)2 had remarkably similar area under the curve (AUC) profiles, suggesting that ~10-fold higher dose should be used for the i.v. relative to the i.a. delivery route for siRNA<(EG18L)2 arthritic knee joint treatments. These trends were confirmed by a peptide nucleic acid (PNA) hybridization assay that enables absolute siRNA quantification in tissue. This measurement showed preferential accumulation of siRNA<(EG18L)2, but not free siRNA, in PTOA knees over contralateral healthy knees, and similar siRNA levels (measured as ng siRNA per mg tissue) when delivered at 10 mg kg−1 i.v. and at 1 mg kg−1 i.a. (Supplementary Fig. 16d). Broader organ biodistribution was also assessed at 30 days following administration of intravenous or intra-articular treatments. While signal remained in all organs, the siRNA<(EG18L)2 signal was highest within PTOA joints of all the tissues analysed (Supplementary Fig. 16).
siMMP13<(EG18L)2 diminishes molecular, histological and clinical manifestations of PTOA
Therapeutic efficacy of siMMP13<(EG18L)2 was tested in a mouse PTOA model after 5 weeks of mechanical knee loading (3 times per week). Mice were treated with siMMP13<(EG18L)2 or siControl<(EG18L)2 (10 mg kg−1) on day 7 and again on day 21 (Fig. 3a). The pressure-pain threshold in PTOA knees was measured by algometer, revealing a 45% reduction in threshold pressure tolerance in siControl<(EG18L)-treated mice, while siMMP13<(EG18L)2 lost only 25% (Fig. 3b). The siMMP13<(EG18L)2 cohort showed relief of joint sensitivity to a level similar to mice receiving intra-articular delivery of the FDA-approved sustained release corticosteroid Zilretta (8 mg kg−1) or 3× per week intraperitoneal treatment with the experimental MMP13-selective small-molecule inhibitor CL-82198 (ref. 19) (10 mg kg−1) (Fig. 3a,b). However, treatment with CL-82198 on the same schedule as siMMP13<(EG18L)2 (once every 2 weeks) produced a less-favourable pain tolerance in PTOA knees, as did treatment with the pan-MMP inhibitor Marimastat (10 mg kg−1 i.p., 3× weekly).
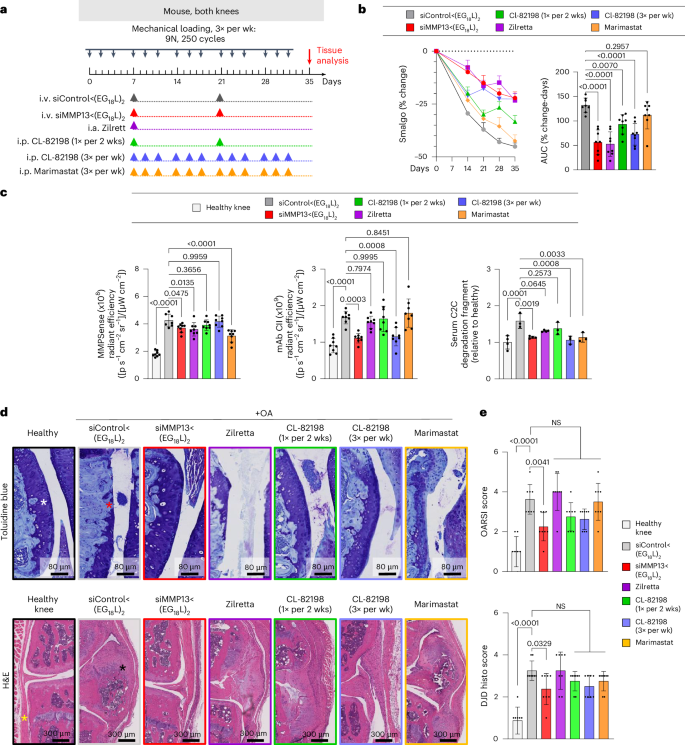
a, Schematic timeline for knee loading and treatment. Bilateral knee loading (3× per week, 5 weeks) was used to induce severe PTOA for comparing therapeutic impact of treatment with i.v. siMMP13<(EG18L)2, i.a. Zilretta, i.p. CL-82198 and i.p. Marimastat. b, Left: mechanical hyperalgesia was measured via algometer through day 35. Data over time displayed as mean + s.e.m. Right: AUC of average Smalgo readings over time was assessed. N = 8. c, Left: total MMP activity in mouse knees was measured using MMPsense 750 Fast (N = 8). Middle: mAbCII binding in knee joints was measured by fluorescence imaging (N = 8). Right: C2C fragments in mouse serum was measured by ELISA (N = 3). d, Knee joints were stained with toluidine blue (top row, femoral condyles shown) and H&E (bottom row, synovium lining and meniscus shown). Asterisks: white, healthy articular surface; red, loss of articular surface; yellow, healthy synovial lining/meniscus; black, synovial thickening/meniscal expansion and calcification. e, Joint cartilage damage was quantitated with the OARSI osteoarthritis cartilage histopathology assessment system (top) and DJD score (bottom). NS, not significant. In all panels, error bars indicate s.d. of biological replicates, unless specified otherwise.
Source data
Pan-MMP activity in PTOA joints was assessed in vivo on study day 30 using MMPSense, an MMP substrate that fluoresces upon proteolytic cleavage. Intravital imaging revealed significantly elevated MMP-driven fluorescence in PTOA knees of mice treated with siControl<(EG18L)2 compared with healthy knees (Fig. 3c). Decreased pan-MMP activity was seen in PTOA knees of mice treated with siMMP13<(EG18L)2, Zilretta and Marimastat, although the MMP13-selective small-molecule inhibitor CL-82198 did not significantly diminish pan-MMP activity in PTOA knees. A fluorescently labelled monoclonal antibody against aberrantly exposed CII collagen (mAbCII) was used to assess relative cartilage damage60,61,62, finding significantly elevated mAbCII localization to PTOA knees (Fig. 3c), thus confirming PTOA-induced cartilage damage in this model. However, mAbCII intensity in PTOA knees was diminished significantly in siMMP13<(EG18L)2-treated samples, approximating the mAbCII signal in healthy knees. Similar trends were observed for serum-based measurements of collagen C2C degradation fragments, a biomarker of cartilage degradation63. PTOA-induced mAbCII signal and serum C2C levels were also diminished by CL-82198 when dosed with higher frequency (3 times per week), underscoring the causative role of MMP13 in PTOA-induced collagen fragmentation and cartilage damage and supporting the hypothesis that MMP13 inhibitory strategies reduce structural OA manifestations. The long-acting steroid Zilretta had little impact on mAbCII binding in PTOA knees or on serum C2C levels, consistent with observations that steroids temporarily control arthritis pain but do not target underlying joint destruction and may even accelerate cartilage thinning64.
Histological analyses of PTOA joints collected on study day 30 revealed profound disruption of cartilage architecture, proteoglycan loss, synovial thickening, osteophyte formation and meniscal mineralization (Fig. 3d and Supplementary Fig. 20). In PTOA knees treated with siMMP13<(EG18L)2, cartilage morphology, proteoglycan content, synovial structure and meniscal anatomy were each relatively maintained, as quantitated using the Osteoarthritis Research Society International (OARSI) OA severity scoring system65,66 (Fig. 3e and Supplementary Table 1). Degenerative joint disease scoring (Supplementary Table 1) also indicated that there were significantly less structural changes in the mechanically loaded joints of mice treated with siMMP13<(EG18L)2 versus those treated with non-targeting siControl<(EG18L)2. Unlike what was seen with siMMP13<(EG18L)2, modest reductions in OA progression and joint degeneration were achieved in PTOA joints treated with CL-82198, although these trends did not reach statistical significance. OA progression and joint degeneration scores were not impacted by treatment with Zilretta or Marimastat.
Using microcomputed tomography (microCT) to quantify osteophytes and ectopic mineralization in PTOA mouse knees, the significant protection conferred by siMMP13<(EG18L)2 against meniscal mineralization and osteophyte formation was confirmed (Extended Data Fig. 5a–c). Importantly, pathologically elevated MMP13 expression in PTOA cartilage, meniscus and synovial compartments was reduced by treatment with siMMP13<(EG18L)2, correlating with decreased presence of degradation fragments (Extended Data Fig. 5d–g). These data show that MMP13 silencing in arthritic joints provides both molecular and clinically tangible benefits, reducing inflammation, clinical pain, articular cartilage loss and secondary manifestations of OA.
MMP13 knockdown in PTOA knees reprograms pro-inflammatory gene expression patterns
To understand the gene expression patterns that underlie the therapeutic outcomes associated with MMP13 silencing, we first measured the impact of PTOA on a small panel of pro-inflammatory genes with previously described roles in OA, including Ngf, Il1b, Tnf and Cdkn2a. These genes were each elevated in mouse PTOA knees compared with healthy knees (Extended Data Fig. 6a). We found that PTOA-induced levels of Ngf, Il1b, Tnf and Cdkn2a were significantly dampened as early as 5 days following i.v. delivery of siMMP13<(EG18L)2 at 10 mg kg−1, gene expression changes that were sustained through day 30. These findings motivated a broader and unbiased analysis of PTOA-induced gene expression changes in knee tissue, along with measuring the broader gene expression impact of MMP13 knockdown. Using a nanoString nCounter Inflammation 254 gene panel, unsupervised analysis of gene expression illustrated extensive PTOA-induced gene expression changes (Extended Data Fig. 6b). Notably, PTOA induction altered signalling along the pathway driven by p38 mitogen associated protein kinase (MAPK), a serine-threonine kinase that phosphorylates a network of substrates that respond to cell stress67. Relevant to OA, p38 MAPK signalling through its substrate MAPK-associated protein kinase 2 (MK2) regulates chondrocyte differentiation, cellular senescence, MMP upregulation and pro-inflammatory cytokine production68. MK2 signalling within human arthritic chondrocytes drives expression of prostaglandin E2, MMP3 and MMP13, leading to arthritic pain, inflammation and cartilage degradation69. Interestingly, we found that the PTOA-induced p38 MAPK gene cluster was the gene enrichment set most potently suppressed in siMMP13<(EG18L)2-treated samples (Extended Data Fig. 6c).
Treatment with siMMP13<(EG18L)2 also significantly suppressed IL-1, chemokine and TNF/NFκB pathway-associated gene clusters (Extended Data Fig. 6c). Notably, these clusters include several genes associated with OA pathogenesis, including those associated with chondrocyte proliferation (Mef2d), synovial fibroblast proliferation (Myc, Jun, Tgfb2, Pdgfa)70,71, angiogenesis (Pdgfba, Flt1, Hif1a), ECM degradation (Mmp3, Masp1), inflammatory signalling through nuclear factor-κB, (Nfκb1, Rela, Relb), cytokines (Il1b, Stat1), chemokines (Cxcl1, Cxcl10, Ccl3, Cxcr4) and toll-like receptors (TLR1, 2, 4, 5, 6 and 8).
Given that many inflammatory and stress response factors induce MMP13 expression and that proteolytic matrix degradation fragments further potentiate inflammation and expression of stress response factors72, these data suggest that siMMP13<(EG18L)2 uncouples a feed-forward inflammation-degradation cycle that drives arthritic joint pathology. These data support this hypothesis, given that selective MMP13 inhibition in arthritic joints suppresses expression of both pro-inflammatory factors and transcription factors that promote MMP13 expression.
K/BxN serum transfer arthritis (STA) multijoint rheumatoid/inflammatory arthritis mouse model
Rheumatoid arthritis is a systemic, multifactorial autoimmune disease that causes painful synovial inflammation and cartilage degradation in multiple joints simultaneously73. Increased MMP13 activity and consequent cartilage loss is a known feature of RA, and daily treatment with an experimental MMP13 small-molecule inhibitor can reduce RA pathogenesis4,74,75,76. Thus, we hypothesized that siMMP13<(EG18L)2 therapy would reduce cartilage loss, decrease induction of pro-inflammatory gene expression and suppress multiple features of RA. We used the transgenic K/BxN serum transfer model to test therapeutic efficacy of siMMP13<(EG18L)2 in an RA-like scenario. In this model, KRN, a T-cell receptor that binds to an endogenous glucose-6-phosphate isomerase (GPI) peptide presented by the IAg7 major histocompatibility complex class II (MHC-II) allele, drives production of anti-GPI autoantibodies and GPI-directed autoimmunity that localizes to articular cartilage/joint tissues. K/BxN mouse serum transiently confers RA to wild-type mouse serum recipients. MMP13 expression is increased in inflamed joints of K/BxN serum recipients, and MMP13-deficient mice are partially protected from K/BxN serum-induced RA76. Carrier-free siRNA delivery in RA has not yet been explored to our knowledge, but siMMP13<(EG18L)2 systemic administration for delivery to multiple afflicted joints is potentially advantageous. Interestingly, albumin-based delivery approaches have been shown to facilitate therapeutic outcomes in RA preclinical studies30,31,48,77,78,79,80,81,82,83,84. Importantly, i.v. delivery of albumin-binding Evans blue to mice resulted in concentrated Evans blue accumulation in arthritic forepaws and hindpaws of K/BxN serum recipients compared with unchallenged mice (Extended Data Fig. 7a,b). Likewise, i.v. delivery of Cy5-labelled MSA led to accumulation in arthritic forepaws and hindpaws of K/BxN serum recipients, but not in unchallenged wild-type mice (Extended Data Fig. 7c). Expression of caveolin-1 and SPARC was elevated in hindpaws of K/BxN recipient mice (Extended Data Fig. 7d). These data confirm increased albumin retention in RA-affected joints, supporting therapeutic testing of albumin-binding siRNA<(EG18L)2 in RA models.
Mice with STA showed accumulation of i.v.-injected Cy5-siRNA<(EG18L)2 within multiple inflamed joints (forepaw, wrist, hindpaw, ankle, knee) (Fig. 4a and Supplementary Fig. 17) at substantially higher levels than what was seen in healthy mice (Extended Data Fig. 7e–g) and exceeding that achieved by treatment with Cy5-siRNA or Cy5-siRNA-Chol (Fig. 4a). Fluorescence microscopy confirmed increased siRNA<(EG18L)2 in fore- and hindpaws of K/BxN recipients, with specific accumulation in soft tissue and cartilage (Fig. 4b). Longevity of siRNA retention was assessed in limbs of K/BXN serum recipients following a single-dose Cy5–siRNA<(EG18L)2. Intravital fluorescence was detected in limbs throughout the 30 days of mice monitoring (Supplementary Fig. 17c), supporting the notion of albumin-mediated delivery and retention of siRNA<(EG18L)2 in multiple afflicted joints in RA models.
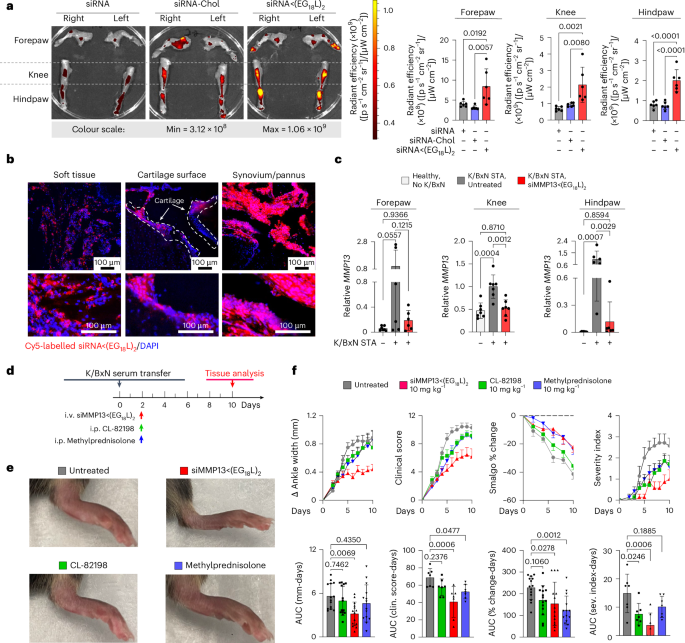
a,b, In a mouse inflammatory arthritis (K/BxN serum transfer) model, mice were treated with Cy5-siRNA<(EG18L)2, Cy5-siRNA-Chol and Cy5-siRNA (1 mg kg−1 i.v.) 4 days after receiving K/BxN serum transfer. Joints of forepaw, hindpaw and knee were collected 24 h later and assessed by IVIS imaging (a) or fluorescence microscopy of hindpaw ankle joints (b). Representative images and quantitation of IVIS data are shown. N = 6. Dashed lines outline articular cartilage in fluorescence microscopy images of tissue cryosections. c, Relative Mmp13 mRNA in forepaws, knees and hindpaws of healthy untreated wild-type mice or in K/BxN serum recipients treated with siControl<(EG18L)2 or siMMP13<(EG18L)2 (10 mg kg−1 i.v.) was measured by RT–qPCR. N = 6. Error bars represent s.d. of biological replicates. d–f, K/BxN serum recipients were treated with siMMP13<(EG18L)2, methylprednisolone or CL-82198 (10 mg kg−1). d, Schematic timeline for K/BxN serum transfer and treatment. e, Photos of hindpaws were collected on treatment day 10, and representative images are shown. f, Ankle width change, clinical score, algometer ankle joint hyperalgesia and severity index were measured for 10 days after treatment. Error bars indicate s.e.m. (top) or s.d. (bottom) of biological replicates.
Source data
siMMP13<(EG18L)2 therapy reduces manifestations and symptoms of RA
Strong Mmp13 induction was seen in forepaws, knees and hindpaws of K/BxN serum recipients compared with healthy mice (Fig. 4c). At treatment day 8 following a single 10 mg kg−1 dose of siMMP13<(EG18L)2, mRNA and protein were decreased in forepaws, knees and hindpaws (Fig. 4c and Extended Data Fig. 8a). Similar to PTOA, MMP13 silencing in this RA model reduced expression of pro-inflammatory genes and cytokines encoding cyclooxygenase 2 (COX2), IL-6, TNF and IL-1β (Supplementary Fig. 18). Clinical parameters of RA were also reduced upon MMP13 knockdown, including ankle swelling, pressure-pain threshold, arthritis clinical score and disease severity index (Fig. 4d–f), and the number of afflicted joints per mouse was decreased (Supplementary Fig. 19). In parallel studies, K/BxN serum recipients were treated with a single dose of Cl-82198 (10 mg kg−1 i.p.) or methylprednisolone (10 mg kg−1 i.p.), neither of which improved ankle swelling (Fig. 4d–f). Although methylprednisolone, but not CL-82198, improved clinical score and pressure-pain tolerance, the effect size was not as pronounced as what was seen with siMMP13<(EG18L)2. Similarly, the disease severity score was somewhat improved upon treatment with CL-82198, but to a lesser extent than what was seen with siMMP13<(EG18L)2. Further, MMP13 silencing resulted in decreased mAbCII binding, indicative of cartilage preservation (Extended Data Fig. 8b) and decreased total MMP activity (Extended Data Fig. 8c).
Histologic examination of toluidine blue-stained cartilage collected on treatment day 8 in K/BxN recipient mice revealed substantial cartilage destruction in hindpaws, knees and forepaws, a phenotype that was diminished by siMMP13<(EG18L)2 treatment (Fig. 5a,b and Extended Data Fig. 9a,b; lower magnification images in Supplementary Figs. 20b and 21–23). Inflammation scoring illustrated similar trends (Fig. 5c and Extended Data Fig. 9c,d). MicroCT studies of bone structure in untreated K/BxN serum recipients revealed erosive bone disease characterized by increased bone surface to volume ratios, decreased bone mineral density (BMD) and decreased bone volume/total volume (BV/TV) ratio (Fig. 5d–g and Supplementary Fig. 23). Remarkably, treatment with siMMP13<(EG18L)2 protected K/BxN serum recipients from inflammation-induced changes in bone surface to volume, BMD and BV/TV measurements. Further, bone erosions characteristic of inflammatory arthritis were evident in forepaws, knees and hindpaws of untreated RA mice, but were minimal or absent in RA mice treated with siMMP13<(EG18L)2 (Extended Data Fig. 9e). In contrast, Cl-82198 and methylprednisolone did not diminish features of erosive bone disease detected by microCT. Serum C2C degradation fragments were significantly elevated in untreated, CL-82198-treated and methylprednisolone-treated K/BxN serum recipients (Extended Data Fig. 10a), while treatment with siMMP13<(EG18L)2 significantly reduced serum C2C fragments. These findings were confirmed using IHC for C1,2C in K/BxN serum recipients’ hindpaw/ankle and knee-joint tissues (Extended Data Fig. 10b).
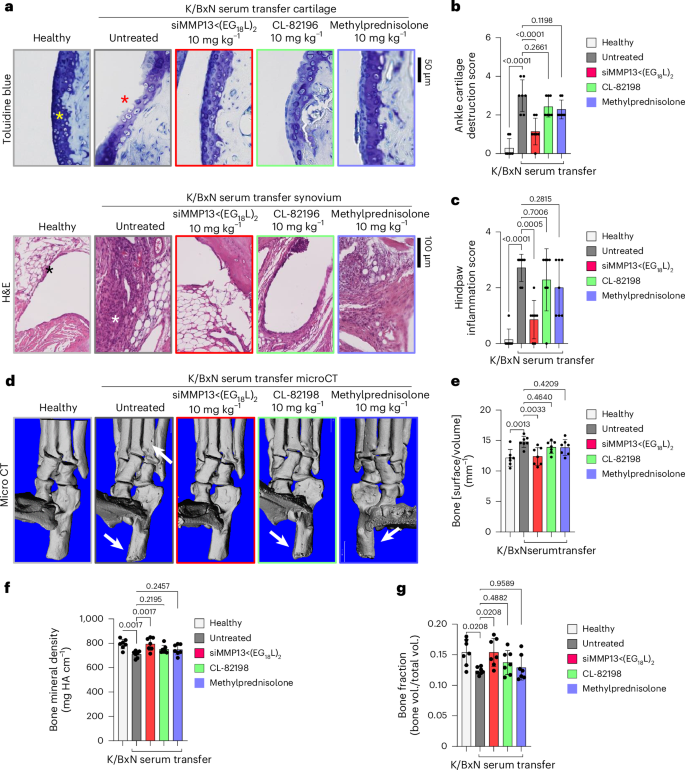
K/BxN serum recipient mice were treated with siMMP13<(EG18L)2, methylprednisolone or CL-82198 (10 mg kg−1). a–c, Histological analysis of toluidine blue-stained ankle cartilage sections (a, top) and H&E-stained hindpaw sections (a, bottom) were used for cartilage destruction scoring (b) and inflammation scoring (c). Asterisks: yellow, articular surface; red, articular surface damage; black, healthy synovium; white, synovial inflammation. d–g, MicroCT-based 3D renderings of hindpaw scans (d, representative images) were used to measure bone surface:volume ratio (e), bone mineral density (f) and bone fraction (g). HA, hydroxyapatite, White arrows in d show bone erosions. All error bars indicate s.d. of biological replicates.
Source data
Hindpaw ankle articular cartilage and synovial RNA was collected from K/BxN serum transfer recipients on treatment day 8 and assessed using the nanoString nCounter mouse inflammation expression panel. These studies identified several gene clusters that were elevated in untreated K/BxN serum transfer recipients compared with healthy mice, many of which were downregulated in samples collected from K/BxN serum transfer recipients treated with siMMP13<(EG18L)2 (Extended Data Fig. 10c). Treated RA animals had gene expression patterns that mirrored the changes seen in PTOA knees upon MMP13 knockdown (for example, IL-1 signalling, NF-kB signalling and chemokine pathways were suppressed by treatment) (Extended Data Fig. 10d). In addition, the pro-inflammatory COX2-encoding gene Ptgs2, the chemokine genes Cxcl5, Ccl7 and Ccl19, and the RA-associated macrophage genes Tnfsf14 and Tr2 were significantly reduced in K/BxN hindpaws treated with siMMP13<(EG18L)2. Thus, the molecular markers of inflammation that characterize RA are dampened by MMP13 knockdown achieved by i.v. delivery of siMMP13<(EG18L)2.
Importantly, serum levels of toxicity markers, including blood urea nitrogen, alanine transaminase, aspartate amino transferase, creatine phosphokinase, lactate dehydrogenase and glucose, were detected within the normal ranges, lacking significant differences between healthy, PTOA or K/BxN serum recipient mice in any of the treatment groups (Supplementary Fig. 24). Liver, kidney, lung, heart and spleen of mice within all groups also showed a normal gross and histological structure (Supplementary Fig. 25).
MMP13 silencing in a Dunkin Hartley guinea pig ACL transection (ACLT) large animal model
ACLT was done on the left knee of Dunkin Hartley guinea pigs (DHGPs) to establish proof of concept in PTOA in a larger species (Fig. 6a). Similar to humans, DHGPs develop OA with aging, with the medial knee compartment showing loss of proteoglycans, fibrillation, cloning of chondrocytes, osteophyte formation and subchondral bone sclerosis85. ACLT is used to mimic PTOA and accelerate the spontaneous OA phenotype86. MMP13 is strongly expressed and highly localized with OA cartilage lesions in DHGPs85. Cy5-siRNA<(EG18L)2 was delivered i.v. on post-surgical day 13 (1 mg kg−1 i.v.), and knees were imaged ex vivo to measure Cy5 fluorescence on day 14, revealing significantly increased Cy5-siRNA<(EG18L)2 accumulation in ACLT knees over unaltered knees (Fig. 6b), while free Cy5-siRNA did not exhibit preferential accumulation in ACLT-damaged knees. Ex vivo Cy5 imaging of organs showed abundant free siRNA in kidneys, while the albumin-binding siRNA<(EG18L)2 was found at highest levels in ACLT-damaged knees and liver, but not in healthy knees or in kidneys (Fig. 6c). Fluorescence microcopy confirmed delivery of Cy5-siRNA<(EG18L)2 but not unconjugated zipper-pattern siRNA to synovia of ACLT-damaged knees, with observable penetration into cartilage (Fig. 6d). Intravenous siMMP13<(EG18L)2 delivered on post-surgical day 7 at 10 mg kg−1 decreased Mmp13 expression in ACLT-damaged knees by >80% (Fig. 6e) and MMP13 protein expression by >70% on day 14 (Fig. 6f,g).
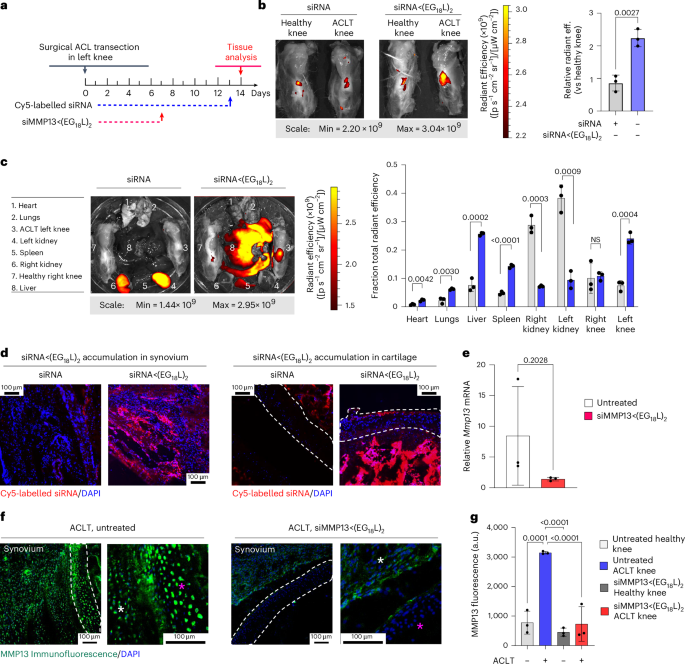
a, Study timeline for ACLT, treatment and sample collection in Dunkin Hartley guinea pigs. b–d, Cy5 fluorescence was measured ex vivo in healthy and ACLT knees (b) and organs (compared using multiple unpaired t-tests with no correction for multiple comparisons) (c) of guinea pigs by IVIS. Tissues were also assessed by fluorescence microscopy of sagittal ACLT knee cartilage/synovial cryosections (d) at 24 h after i.v. delivery of Cy5-siRNA or Cy5-siRNA<(EG18L)2 at 1 mg kg−1. Representative images are shown. In d: blue, DAPI; red, Cy5 siRNA. e, Guinea pig Mmp13 mRNA expression (ACLT/healthy knee) as determined by RT–qPCR. f,g, MMP13 immunofluorescence in cartilage/synovial cryosections of ACLT knee joints that were untreated (left) or treated with siMMP13<(EG18L)2 (10 mg kg−1 i.v.). Asterisks: white, synovium; magenta, femoral condyle cartilage. Representative images are shown with dashed lines outlining articular cartilage (f). MMP13 immunofluorescence intensity was quantitated using morphometric software (g). All error bars indicate s.d. of biological replicates. Knees labelled healthy indicate a contralateral limb (right) without ACL transection (non-surgical).
Source data
Outlook
A unique albumin-binding RNAi conjugate (siRNA<(EG18L)2) was engineered for MMP13-selective gene targeting in the context of arthritis. This optimized siRNA–lipid conjugate was capable of preferential accumulation and long-term retention within arthritic joints, robustly decreasing arthritis-induced MMP13 and interrupting the forward-feeding cycle of inflammatory joint damage to limit arthritis progression. Overall, this albumin-binding siRNA-L2 system shows promise as a systemic and/or local anti-MMP13-specific therapy. This siRNA delivery technology is readily amenable to modular engineering for targeting other arthritis disease-driving genes and/or gene combinations. Beyond the scope of arthritis, we believe this technology may be utilized to deliver to other diseased or injured tissues characterized by inflammation, including tumours, tissues undergoing ischaemic injury and bone fractures.