Currently, a weekly SC MTX injection syringe/pen product is commercially available in approximately 50 countries and regions17, because SC administration of MTX is known to be better absorbed and tolerated clinically and may be more effective than oral MTX at higher doses15.
A highly variable (25–100%)5 BA of oral MTX and large individual differences in therapeutic efficacy/side effects occurs by saturable intestinal absorption via drug carrier-mediated transport (reduced folate carrier 1) and nonlinear pharmacokinetics of MTX, which may contribute to the GI side effects6,7,8,9. For example, in the comparison between SC and PO, gastrointestinal disorders were 15.9% versus 19.7%20 and 15.4% versus 34.0%21; nausea was 55% versus 34%22 and 5.8% versus 8.5%20, 3.8% versus 14.0%21, 16.6% versus 12.2%15, and 37% versus 63%23; vomiting was 8% versus 7%22, 2.9% versus 4.2%20, 3.6% versus 3.2%15, and 11% versus 30%23; indigestion was 6.7% versus 5.9%15, 29% versus 48%23, and 7.3% versus 3.2%15; abdominal pain was 8.8% versus 10.6%15 and 0% versus 1.4%20; diarrhea was 12% versus 23%22, 2.6% versus 6.9%15, 0% versus 2.8%20, and 0% versus 6.0%21. As such, for most adverse effects, oral MTX therapy resulted in more adverse GI effects than SC MTX therapy.
However, SC injections are painful, patients are fearful of the procedure, which requires learning the injection technique, and syringe disposal is also a medical problem due to issues of disposal costs and burdens at facilities such as hospitals/pharmacies, along with the risk of infection of medical staff. The main reasons patients avoid injection therapy include a fear/dislike of needles and injections. A meta-analysis reported that 16% of adults avoided influenza vaccination due to fear of needles and injections. In the case of pneumococcal vaccination, a nationally representative sample of adults in the USA showed that 20% did not receive tetanus vaccination because they disliked needles24,25. A survey of 100 physicians in the USA found that 71% responded that fear of needles was a contributing factor to not receiving tetanus vaccinations. These figures were 71% for influenza vaccinations and 69% for pneumococcal vaccinations24,25. In a nationally representative survey of individuals aged 65 and older in the USA, 2.6% did not receive the pneumococcal vaccine because they “did not like injections or needles”24,26. In a study of patients with RA comparing prefilled pens with refilled syringes, pain at the injection site was more common with pens, while redness was more common with syringes, although both devices were generally well tolerated27.
In this study, we focused on the needleless PJI18 as a novel MTX administration device in clinical practice to develop a new painless, patient-friendly, easy administration method. In the past, PJIs with controllable jet pressure were used in a phase I study of intradermal COVID-19 DNA19. The present study was conducted to assess the safety and immunogenicity of an intradermal coronavirus disease 2019 (COVID-19) DNA vaccine (AG0302-COVID-19) administered using a PJI and nonrandomized, open-label phase I study design. Regarding immunogenicity, a cellular immune response was observed in some study participants after AG0302-COVID19 intradermal inoculation, but no significant antibody production was observed. Overall, no notable safety issues were observed with the intradermal inoculations of AG0302-COVID19, and therefore this study demonstrated that intradermal administration via the PJI is safe with no specific safety concerns. In a previous clinical study19, the PJI was applied as a method of administering vaccine preparations intradermally. Since immune cells (antigen-presenting cells) are localized in the skin, intradermal administration is used to efficiently acquire immunity.
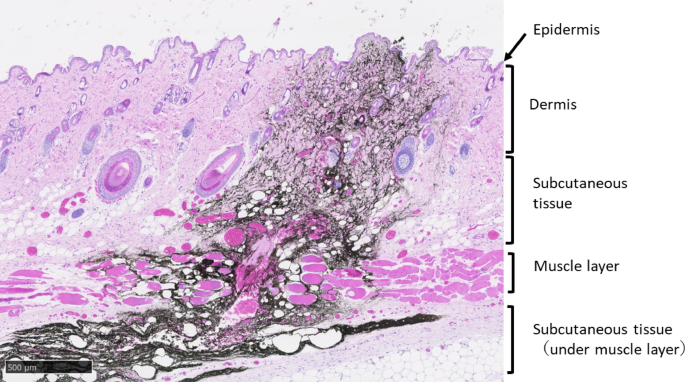
In this study, MTX, an autoimmune disease treatment drug, was used, and its usefulness in intradermal administration was evaluated. However, commercially available RA treatment drugs are subcutaneous injection preparations. Therefore, in this study, although using rats, we compared PK and BA across various administration methods (IV, PO, SC) to evaluate potential SC administration via the PJI. The results showed PK parameters and BA similar to those after SC administration, suggesting the potential of the PJI for SC administration in rats. To date, it has not been reported whether the PJI can administer drugs subcutaneously. Therefore, we first investigated the dispersion pattern of black ink via the PJI to determine where the ink reaches the skin and/or subcutis. To the best of our knowledge, this is the first report showing that black ink injected on the skin surface of rat flanks using the PJI is extensively and rapidly distributed in the subcutaneous tissue immediately after administration and reaches deep into the subcutis (Fig. 1). Therefore, this PJI administration method is thought to inject to the same skin depth as SC injection, that is, the drug can penetrate into subcutaneous tissue including the muscle layer. Second, we investigated the PK and BA of MTX in rats after four different administration methods: IV, PO, SC, and PJI. The PJI is in principle the same administration route as SC. In the comparison between PO and parenteral administration (IV, SC), the PK results in this study were similar to those in previous studies, showing that parenterally administered MTX led to more rapid, complete absorption and higher serum levels than PO administration8,9,10. The comparison between PJI and SC administration showed that there were no statistically significant differences in the PK and BA of MTX (Table 1, Figs. 2 and 3). For each PK parameter, Cmax was slightly higher in the PJI than in the SC group, although the differences were not statistically significant. In addition, the plasma concentration of MTX (mean ± SD) at 0.17 h in the PJI group (1.49 ± 0.04 µg/mL) was significantly higher than that in the SC group (0.77 ± 0.18 µg/mL, p < 0.05). These differences in Cmax at 0.17 h were likely due to differences in injection power, as the PJI injects the agent using the power of the gas explosion gas generator. MRT0–∞ with the PJI was slightly shorter than that with SC administration, although not a statistically significant difference. This might be due to a larger drug absorption rate constant (ka) because the PJI uses the power of the gas explosion to inject MTX.
The absolute BA in the PO, SC, and PJI groups compared with the IV group was 30.6%, 99.1%, and 100.8%, respectively. The AUC0–∞ after PO was comparatively low, and therefore the absolute BA was lower than that in the IV group in this animal study. It is known that the BA of low-dose oral MTX varies greatly among RA patients, ranging from 25 to 100%5, and there are significant individual differences in the absorption of oral MTX from the gastrointestinal tract, which result in variable serum concentrations28.
The relative BA after PJI compared with SC administration was 101.7%. Notably, the AUC0–∞ and absolute BA after PJI and SC had nearly the same values as after IV administration. In addition, although not a statistically significant difference, absolute BA with the PJI tends to be slightly greater than with SC administration, which may be due in part to MTX absorption from the skin with the PJI via the muscle layer under subcutaneous tissue. Therefore, this is the first report that a low-molecular-weight molecule such as MTX demonstrated rapid distribution over a wide SC tissue area including the muscle layer under that tissue after the PJI was injected into rat flank skin surface, with the same BA as after SC injection.
This new PJI method has several benefits for patients compared with the usual SC injection formulation (syringe/pen). First, patients do not feel fear or pain when receiving the drug because the PJI has no needle. Second, while ordinary SC injection syringes and injection kits result in medical waste, the containers of drug products using the PJI are not medical waste. Finally, even patients with hand or finger disabilities might easily use the PJI by themselves compared with the present SC injection formulation (syringe/pen). Thus, these results suggest that a painless, patient-friendly drug administration formulation of MTX using the PJI may be useful for self-administration therapy for RA patients in clinical settings.
This study had several limitations in considering the application of this PJI method to humans. First, we investigated the diffusion pattern and penetration depth of black ink in the skin and/or subcutaneous tissue of rats via the PJI (Fig. 1) in this study. The results showed that administration via the PJI may reach depths beyond the SC layer of rats. These results suggest that a portion of the MTX dose may be administered intramuscularly in rats, and that PK parameters may change compared with SC administration. However, as shown in Figs. 2 and 3 and Table 1, the PK parameters obtained with PJI administration were similar to those obtained with SC administration, suggesting that the MTX administration method using the PJI is essentially equivalent to SC administration. It should be noted that the results in this study were obtained in rats using a PJI device designed for rats (Actranza® lab.). Because human and rat skin differ significantly in thickness and texture, it is unclear whether the results of this study can be extrapolated to SC administration in humans. Therefore, we consider that pig or human skin, which are more commonly used as nonclinical trial models for transdermal administration due to their similarity in skin thickness and texture, or clinical trials in humans, are necessary to ultimately verify the potential of MTX SC administration using a PJI device designed for humans. Second, it was a fixed-dose animal study, and the MTX dose used was very high at 2.5 mg/kg, compared with the usual dose of MTX for the treatment of RA in humans (i.e., 7.5–15 mg once weekly)17,29. The possibility that high MTX dosage may mask differences between administration methods was not examined. In the future, it will be necessary to examine differences in BA between the PJI and other administration methods using several MTX dosages that take clinical dosages into consideration. Third, this was only a pharmacokinetic study to examine BA, not a bioequivalence (BE) animal study. Since it was difficult to conduct a BE study in rats, we evaluated BA using a parallel-group design in this study. Given the small sample size of 3–4 rats used in this study, there is a possibility that the power was insufficient. When interpreting the results of this study, this point should be noted. Further studies with larger sample sizes or BE studies using crossover trial designs in other animal species, such as pigs or humans, are necessary. Fourth, we do not have the safety data on the potential damage of local tissue after high-pressure injection via the PJI. However, it was reported that in a rat model to evaluate the usefulness of the PJI as a DNA vaccine tool, histopathological analysis of skin damage caused by the PJI was performed at 0, 6, and 24 h after Luc-plasmid DNA injection, showing that PJI administration caused small spherical cleavages and swelling that were observable up to 6 h postinjection. Those small spherical cleavages and swelling disappeared by 24 h. No other remarkable intradermal inflammation or bleeding was observed at any observation time point. Furthermore, no remarkable adverse events were observed at 24 h after PJI administration. These results show that the PJI did not induce skin damage complications after plasmid DNA injection or bleeding at the injection site30. In another clinical study19, the PJI was also reported to be safe in a human. The data mentioned above did not include safety data on PJI preparations of MTX in human RA patients Therefore, we need to pay attention to the safety of PJI preparations with MTX in human RA patients, due to interspecies differences. Accordingly, further BE studies in humans are needed to demonstrate BE, including safety, between PJI and SC administration using the METJECT subcutaneous syringe/pen. Fifth, the effects of drug formulation characteristics (drug concentration, volume, excipients, etc.) on PJI performance were not examined in this study. In the case of drug solutions that are suspensions, particle size and viscosity may affect injection behavior. However, the MTX solution used in this study was a nonviscous aqueous solution, and the drug volume administered was 50 µL, which is the standard container unit volume. Therefore, it was considered unlikely to affect the performance of the PJI. Consequently, the MTX solution used in this study can be administered via the PJI without issues, similar to existing clinical formulations, and concerns regarding performance degradation due to drug concentration or additives were limited in this study. However, in the future, if high-concentration drug solutions, large volumes, or viscous additives (excipients) such as absorption enhancers are used, it will be necessary to evaluate the potential impact of these drug formulations on PJI performance. Sixth, since PJI devices are not currently commercially available, it is not possible at this time to compare their cost to that of existing MTX injections. However, this device can be used repeatedly, and consumables are limited to drug-filled container units. Therefore, initial costs are concentrated on the purchase cost of the device itself, and subsequent costs depend solely on the drug container units. If appropriate pricing is established for the drug container units, it is expected that cost–benefit comparable to or exceeding that of existing injectable formulations can be achieved. Additionally, previous studies reported that patients demonstrate greater willingness to pay for administration methods that offer reduced pain associated with injection, improved operability, enhanced drug efficacy, and improved side effect profiles27. Finally, this study was an animal experiment using rats, and at present, no data on fear responses associated with sound or pressure stimuli and ease of use for patients have been obtained. These data need to be collected as future research topics. Although the use and operability of this product may require a certain amount of skill and practice, the injection button is flexible and can be pressed without excessive force. It can be used simply by replacing the disposable container unit (cartridge) filled with the drug solution, and attachment and removal are relatively easy. However, depending on the degree of joint dysfunction in RA patients, it may be difficult to operate the PJI or replace the container unit. Therefore, it is essential to evaluate the operability based on the degree of joint dysfunction in RA patients. In addition, prior education and training are required to ensure proper use. These measures are expected to increase the feasibility of administration by RA patients and caregivers by reducing patient anxiety and pain and avoiding the risk of needle-stick injuries and drug exposure for caregivers. Therefore, the PJI is suggested to have greater clinical utility than conventional formulations. Overall, SC administration using the PJI is expected to contribute to improved tolerability, medication adherence, and treatment continuity. Furthermore, enabling self-administration at home may also contribute to improved treatment outcomes and patient satisfaction with quality of life (QOL). However, currently, there are no data available for humans, particularly RA patients. Going forward, it will be necessary to collect such data through clinical trials during the clinical development process and utilize it to improve the PJI device.
By resolving the above limitations in this study, in the near future this MTX administration method using the PJI could avoid several problems of SC preparations, including painful injections, fear of the procedure, the need to learn the injection technique, syringe disposal from the viewpoint of disposal costs and burdens at hospitals/pharmacies, and the risk of infection of medical staff.
The results of these study findings may provide physicians and patients with the opportunity to select the most appropriate administration method based on individual circumstances and issues, such as patient preferences, medication adherence, quality of life, and drug costs. Considering these factors, the PJI is one of the most patient-friendly administration methods among those currently available.
In conclusion, this study demonstrated that MTX administration using the PJI may ensure BA in rats that is nearly equivalent to that of SC administration. A pain-free, patient-friendly MTX administration formulation using the PJI may be useful for self-administration therapy in RA patients in clinical practice. However, further studies are needed to verify BA or BE in humans by conducting BA or BE tests in animal species with skin similar to that in humans or in humans themselves.