Some people with rheumatoid arthritis (RA) take a disease-modifying antirheumatic drug (DMARD) and/or a biologic and reach remission. Others try drug after drug after drug and have yet to find one that’s effective — or that treats their symptoms without causing intolerable side effects. At the same time, other patients with active rheumatoid arthritis opt not to take the most powerful and effective medications on the market or can’t take them because of their medical history or personal risk factors that would make doing so dangerous. There is growing interest in exploring alternative approaches, such as stimulating the vagus nerve to treat rheumatoid arthritis.
While many scientists are continuing to explore better drug options, some are focused on developing new treatments that aren’t medications at all. One such experimental approach that’s gaining some traction is called neuromodulation, which entails using electrical impulses to stimulate nerves that regulate inflammation in the body.
What Is the Vagus Nerve — and What Does it Have to Do with Rheumatoid Arthritis?
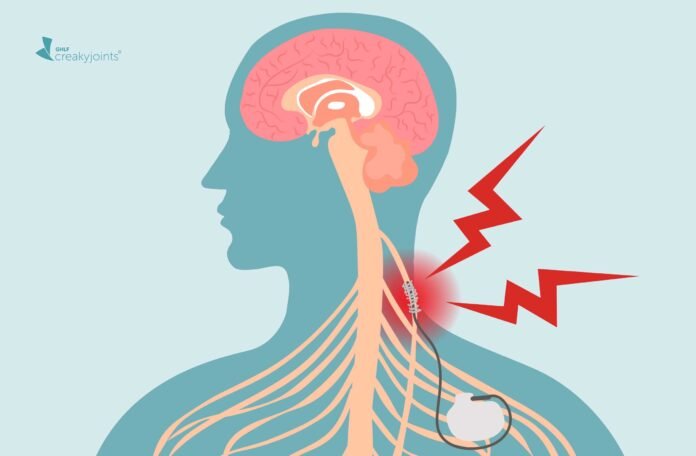
The longest of the cranial nerves, the vagus nerve runs from the brain, through the face and neck, and down into the abdomen. It’s a key part of the autonomic nervous system, the division of your nervous system that controls functions you don’t have to think about, such as breathing, digesting food, and the beating of your heart. The vagus nerve is also the home of the inflammatory reflex, a pathway that appears to be crucial for detecting and modulating inflammation.
When something in your body gets injured or attacked by an invader (like a virus or bacteria), the vagus nerve and the inflammatory reflex help decide how strong of an immune response the body should mount. When that response is appropriate, germs get killed off and injured tissue starts to heal. But when it’s too aggressive for the threat at hand — imagine trying to put out a small candle by holding it under a waterfall — you end up with chronic inflammation, which results in joint and tissue damage.
If you have an autoimmune or inflammatory condition, you already know that your immune system is far more revved up than it should be. In the case of rheumatoid arthritis, the immune system mistakenly tries to attack healthy tissue in the joints as well as in other organs. No one knows exactly what sets RA in motion, but experts do know that inflammatory substances called cytokines play an important role in causing tissue damage. Those cytokines include tumor necrosis factor (TNF) and various interleukins (IL), among others.
Scientists now know that the vagus nerve has the power to reduce the production of these cytokines.
It’s worth noting that the vagus nerve also modulates the body’s stress response and is integral to the mind-body connection, says Vibeke Strand, MD, Adjunct Clinical Professor in the Division of Immunology/Rheumatology at Stanford University and a pharmaceutical and biotech consultant.
This overlap isn’t just an interesting coincidence. “The autonomic nervous system is part and parcel of our immune response,” says Dr. Strand. “It takes a little time for some people to wrap their heads around that.”
While stress alone doesn’t directly cause inflammatory conditions like RA, it can certainly exacerbate them and lead to additional immune system overactivity as modulated by the vagus nerve, Dr. Strand adds.
Emerging research suggests that stimulating the inflammatory reflex — such as by sending electrical impulses to the vagus nerve — has the potential to treat RA by reducing the body’s production of inflammatory cytokines.
Neuromodulation vs. RA Medication: Same Targets, Different Approach
Many people with RA take medications that target specific cytokines. Biologic medications such as adalimumab (Humira), certolizumab pegol (Cimzia), etanercept (Enbrel), golimumab (Simponi, Simponi Aria) and infliximab (Remicade) target the ctytokine TNF, whereas anakinra (Kineret) targets IL-1 and sarilumab (Kevzara) and tocilizumab (Actemra) target IL-6.
But these therapies don’t work for everyone with RA. For these patients, neuromodulation may be another way to reduce the production of cytokines that cause inflammation. If ongoing and future studies are positive, neuromodulation might one day become a viable option for RA patients who don’t respond to medication, can’t take it, or don’t want to, says Dr. Strand. It might also be used in conjunction with drug therapies to boost their effectiveness.
Several researchers and biotech companies are currently investigating neuromodulation for RA. Most are focused on stimulating the vagus nerve.
“The premise is that when you stimulate the vagus nerve it leads to the production of acetylcholine [a neurotransmitter], which binds to receptors on cells that secrete cytokines. When acetylcholine binds to those cytokine-producing cells, it inhibits them from producing TNF and interleukin-6,” says rheumatologist Mark Genovese, MD, Emeritus Professor of Medicine at Stanford University.
Dr. Genovese conducted a pilot study on vagus nerve stimulation that was presented at the 2019 European Congress of Rheumatology (EULAR) conference. The full version of the same research, sponsored by bioelectric company SetPoint Medical, was published in the journal, The Lancet Rheumatology, in 2020.
In the study, 14 people with RA who had tried and did not adequately respond to at least two medications had a small “MicroRegulator” — about the size of a nickel — implanted on the left side of the neck along the vagus nerve. Although the primary goal of this small trial was to assess the safety of the device, half of patients who had impulses sent to the MicroRegulator once a day had significant improvements in their RA disease activity scores. A decrease in cytokine production was also measured.
A few adverse effects were reported, but all were temporary. Those included pain and swelling at the incision site as well as one patient who had temporary vocal cord paralysis.
The RESET-RA trial was a key clinical study testing the SetPoint System, a new device designed for people with moderate-to-severe RA who did not improve with other treatments. In the study, participants had a small device implanted on the vagus nerve to deliver gentle electrical stimulation once a day, helping activate the body’s natural anti-inflammatory response. Results showed that the therapy was both safe and effective, meeting its main goal and providing lasting improvements in RA symptoms over time.
Neuromodulation Moves from Research to Reality
In July 2025, the FDA approved the SetPoint System — the first neuromodulation device cleared for treating rheumatoid arthritis. The SetPoint System is a small, implantable device that delivers gentle electrical stimulation to the vagus nerve once a day. By activating the body’s natural anti-inflammatory and immune-restoring pathways, it offers a way to calm inflammation without the immune-suppressing risks of many current RA medications. It’s intended for adults with moderate-to-severe RA who haven’t had success with, or can’t take, biologics or other advanced therapies. Approval was based on results from the large RESET-RA trial, which showed meaningful improvements in disease activity and a strong safety profile. The therapy is expected to launch in select U.S. cities this year, with broader availability planned for 2026. Learn more here.
Why this matters for patients:
This approval marks an important new option for people with RA — offering hope for better control of symptoms when standard treatments haven’t worked.