Human subjects research
This study does not involve sex-based analysis, and sex was not considered in the study design, since RA involves both. Therefore, for human participants, both male and female patients were included. All patients included in this study signed a written informed consent form for the use of abandoned specimens (meniscus, cartilage, synovium, and synovial fluid) and publication of any potentially identifiable images or data, as approved by the Ethics Committee of Sun Yat-sen Memorial Hospital (2009-06, 2020-126). We included patients with RA who met the 1987 American Rheumatism Association criteria40 and the 2010 ACR/EULAR criteria41.
For patients with RA who underwent systemic treatment without remission of severe knee pain or knee deformity (knee varus or knee valgus), we performed knee arthroplasty and obtained meniscus, cartilage, synovium, and synovial fluid samples. For patients with OA who met Kellgren-Lawrence III or IV grading42 and had severe knee pain that could not be relieved by NSAIDs, we performed knee arthroplasty and obtained knee tissue samples. For the control knee synovium samples, we obtained normal knees by thigh amputation or hemipelvectomy. For RA shoulder and hip joint synovia, we obtained them by arthroplasty surgery. For RA elbow, wrist, and ankle joint synovia, we obtained them by arthroscopic surgery. For RA first metatarsophalangeal joint synovium, we obtained it by Parker-Pearson fine needle biopsy. Radiological data from patients with OA and RA were used for radiological analysis, and samples from normal, OA, and RA knees were used for histological analysis, single-cell RNA sequencing, cell cytometry, cell culture, in vitro and in vivo experiments, and transcriptomic RNA sequencing.
The cohort was established in 2013 for long-term follow-up in RA cohort studies. All patients with RA underwent knee Parker-Pearson fine needle biopsy at the onset of knee symptoms (knee pain and swelling) to obtain knee synovium tissue samples. After the biopsy, all patients underwent systematic antirheumatoid treatment. By the end of May 2024, 16 female and 1 male patients completed the follow-up period (detailed in Supplementary Table 1). We categorized the patients into non-TKA and TKA groups based on whether they underwent TKA. For the TKA group, the follow-up endpoint was set at the time of surgery; for the non-TKA group, the follow-up endpoint was set at May 2024. Knee joint function was assessed using the KSS score29 for both groups.
Radiological analysis of human knee data
An X-ray in the anteroposterior view in the standing posture was used to measure the medial joint space and the lateral joint space by selecting the minimum segment between the medial and lateral compartments43. All measurements were based on a real-world plotting scale. MRI with a T2-weighted sequence was used for knee and wrist menisci measurement. In the knee coronal sequence, the image plan in which the medial tibial spine volume was maximal was selected for measuring the thickness and width of the meniscal body segment, whereas in the sagittal sequence, the image plan on the midpoint of the medial/lateral compartment was selected for measuring the thickness and width of the medial/lateral meniscal anterior and posterior horn44.
Histological morphology analysis and scoring of human synovium and meniscus
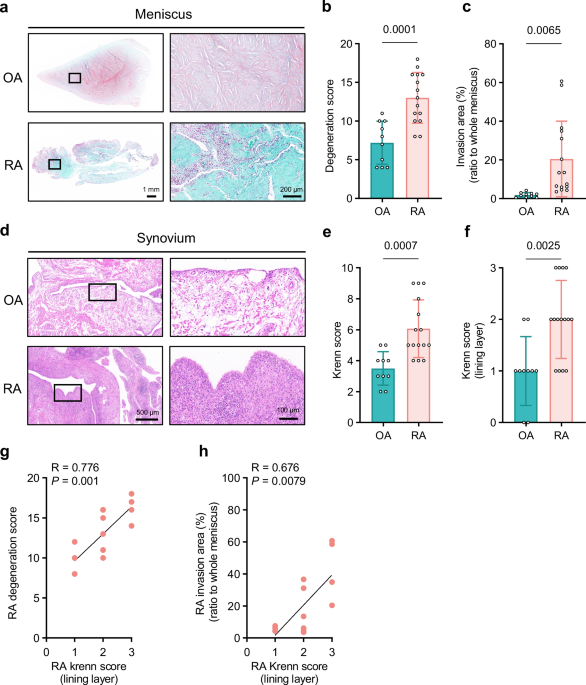
For histological sections, fresh meniscus, cartilage, and synovial tissues obtained from knee arthroplasty surgery (for RA and OA subjects) were fixed in 4% paraformaldehyde and embedded in paraffin. Human knee synovial fluid was centrifuged at 770 g for 5 min, and the deposit was fixed in 4% paraformaldehyde and embedded in paraffin. All paraffin-embedded specimens were dissected into 5 μm sections and stored at room temperature. Sections of the human synovium and meniscus were stained with haematoxylin and eosin (HE), and Safranin-O-Fast Green. The lining (cell) layer of the synovium was identified on HE staining as the membrane facing the joint cavity, characterised by ellipsoidal cells arranged in layers45. Synovial sections were scored using the total Krenn synovitis score (ranging from 0–9 points) and the enlargement of the synovial lining cell layer component of the Krenn synovitis score (ranging from 0–3 points)19.
Meniscal sections were scored using the Pauli degeneration score17 (ranging from 0–18 points). Synovium invasion area on the meniscus was defined as the area containing limited clustered or widely infiltrated cells, usually accompanied by new angiogenesis. The area was calculated as the ratio of the invasion area to the total meniscal area using ImageJ software (version 1.54).
Immunohistochemistry and mIHC
For immunohistochemistry (IHC), antigen retrieval was performed at pH 9.0 using Tris-EDTA for 20 min by microwave heating. Sections were incubated with primary antibodies of CD142 (Bioss, 1:200), PRG4 (Abcam, 1:200), THY1 (Proteintech, 1:200), and ABCC4 (Proteintech, 1:200) at 4 °C overnight, followed by incubation with anti-rabbit horseradish peroxidase (HRP) secondary antibody (Servicebio, 1:200) at room temperature for 50 min. HRP staining was performed using DAB Peroxidase HRP Substrate (Servicebio).
For mIHC, antigen retrieval was performed at pH 9.0 using Tris-EDTA for 20 min by microwave heating. The main steps of mIHC are as follows: sections were incubated with primary antibody at 4 °C overnight, and then with anti-rabbit HRP secondary antibody at room temperature for 50 min, followed by tyramide signal amplification (TSA) staining at room temperature for 10 min. Antigen retrieval was performed again for the next primary antibody incubation. Primary antibodies against PRG4 (Abcam, 1:1000), CD146 (Proteintech, 1:1000), CD142 (Cell Signaling Technology, 1:600), and THY1 (Abcam, 1:600) were incubated. IF440-TSA (anti-CD146, Servicebio, 1:500), iF488-TSA (anti-CD142, Servicebio, 1:500), iF555-TSA (anti-PRG4, Servicebio, 1:500) and iF647-TSA (anti-THY1, Servicebio, 1:500) were used for TSA staining. DAPI staining (Servicebio) was performed last to label nuclei.
Quantitative and spatial analysis of mIHC
Human synovial sections stained with mIHC were scanned using Akoya whole-slide multispectral imaging in fluorophore Spectral DAPI (excitation: 368; emission: 461), Opal Polaris 480 (excitation: 450; emission: 500), Opal 520 (excitation: 494; emission: 525), Opal 570 (excitation: 550; emission: 570), and Opal 690 (excitation: 6676; emission: 694). Three fields (923 μm × 692 μm) in each section were chosen for analysis, and their data were summed as one sample. Akoya InForm (version 1.6) loaded the chosen fields to output phenoptic data, followed by tissue segmentation, cell segmentation, and phenotyping. For tissue segmentation, the lining and sublining were segmented using haematoxylin and corresponding HE staining. For cell segmentation, the minimum nuclear size was set at 10, the cytoplasm thickness was set at 5, and the membrane search distance was set at 10. For cell phenotyping, 20 cells for each fluorophore were manually chosen for classifier training, and the classifier was used for field phenotyping to label each segmented cell. The R package PhenoptrReports (version 0.3.3) processed the phenoptics data and calculated all phenotyping cells in the LL and SL of each section and the nearest distance between each pair of phenotyping cells. The results from three fields were summed and averaged for each sample.
Human meniscus, cartilage, and synovium tissue processing for cell suspensions and cell culturing
Fresh meniscus, cartilage, and synovial tissues were obtained from knee arthroplasty (for RA and OA subjects) and thigh amputation or hemipelvectomy (for control subjects). For flow cytometry, cell culturing and RNA sequencing, fresh synovium tissues were dissected in 2 mm pieces and then digested in collagenase I solution (2 mg/mL, Gibco; +10% FBS, Gibco; +1% penicillin-streptomycin, Gibco) for 2 h in 37 °C, after which solution was filtered by 70 nm cell strainer (BIOFIL) and centrifuged at 430 g for 5 minutes to obtain cells and made into single cell suspensions. Meniscal and cartilage tissues were dissected into 2 mm pieces and then digested in collagenase P solution (2 mg/mL for meniscus and 0.25 mg/mL for cartilage, Roche; +10% FBS, Gibco; +1% penicillin-streptomycin, Gibco) for 6–8 h at 37 °C for cell suspensions.
For human primary meniscal cells and SF, we cultured them in Dulbecco’s modified Eagle’s medium/Hams F12 (DMEM/F-12, Gibco) with 10% FBS and 1% penicillin-streptomycin in an incubator at 37 °C and 5% CO2.
Flow cytometry and synovial fibroblast collection
For the flow cytometric analysis of human synovial cells, red blood cell lysis buffer (Solarbio) was added to the cell suspensions for 10 min at room temperature, and the suspensions were centrifuged, washed, and resuspended. After removing red blood cells, synovial cells were stained with antibodies against CD31 (BV421, BioLegend), CD45 (APC, BioLegend), CD142 (PE, BioLegend), and 1% BSA in PBS for 20 min at room temperature. 7-Aminoactinomycin D (7-AAD, BioLegend) was added to cell suspensions, and cells were passed through a 100 μm filter. Data were acquired using BD FACSverse and analysed using FlowJo (version 10.8). Based on the above protocol, we sorted CD31–/CD45– living fibroblasts from primary human synovial cells and collected CD142+ and CD142– fibroblasts using BDJAzz for further in vivo and in vitro experiments.
Generation and analysis of single-cell RNA sequencing data
Fresh meniscus, cartilage, and synovial tissues obtained from the same RA knee by TKA were made into cell suspensions, in which meniscal cells, chondrocytes, and synovial cells were captured with 10x Genomics based on the Illumina NovaSeq 6000 platform provided by NovelBio Bio-Pharm Technology Co., Ltd. Fastp was applied with a default parameter for filtering the adaptor sequence and removing low-quality reads46. Umi tools were used for single-cell transcriptome analysis to identify the cell barcode whitelist, extract the cell barcode UMIs, and calculate the cell expression counts based on the filtered clean FASTQ data47. Downstream analysis was performed using the Seurat R package (version 4.0.2) as follows: cells with >20% mitochondrial reads, <200 genes, or <200 UMI were excluded from the analysis. We merged cells from the meniscus, cartilage, and synovium, and the per-cell counts were normalized and scaled. The fastMNN function (k = 5, d = 50, approximate = TRUE) in the R package scran (version 1.12.1) was used to apply the mutual nearest-neighbor method to correct for batch effects among samples. The first ten principal components were retained for the UMAP projection. GraphCluster and K-means were used for cell clustering (resolution set at 0.8 for the full analysis), and the Wilcoxon rank-sum test was used for marker gene analysis (min.pct = 0.1, logfc. threshold = 0.25). We identified the meniscal cells as COL1A1+/CEMIP+, chondrocytes as ACAN+/COL2A1+, SF as PRG4+/COL1A1+, macrophages as CD14+/LYZ+, smooth muscle cells as MYL9+/NOTCH3+, T cells as CD3D+/CD8A+, endothelial cells as MCAM+(CD146+)/PECAM1+, and mast cells as CD79A+/TPSB2+. SF were re-analysed to identify subclusters in the fastMNN-based UMAP space with a resolution set at 1.8, and marker genes were calculated (min.pct = 0.1, logfc. threshold = 0.25). For public scRNA-seq data projection, public scRNA-seq data were downloaded according to the reference guidance. R package scmap (version 1.24.0) was used. Fibroblast subsets of public data were set as referenced clusters by indexCluster, and mapped with the current fibroblast data of this study by scmapCluster, projecting to the same UMAP space. GO and Gene Set Enrichment Analysis (GSEA) was completed by R package clusterProfiler (version 4.10.0) and visualized by R package GseaVis (version 0.1.0).
Cell-to-cell interaction analysis
After identifying the three subclusters of SF, we performed a cell-to-cell interaction analysis using the R package CellChat (version 1.6.1). We created a CellChat object with clusters of meniscal cells and chondrocytes and three subclusters of SF. CellChat database for humans (CellChatDB.human) was loaded, and the “Secreted Signaling” category was chosen for analysis. Overexpressed genes and interactions were identified and projected onto the protein-protein interactions (PPI) of the CellChat object. We then computed the communication probability based on the algorithm’s truncated mean (cutoff = 20%) and filtered out the cell-to-cell communication if there were <10 cells in certain cell groups, which were aggregated into the final cell-to-cell communication network. Three subclusters of SF were set as communication senders, while meniscal cells and chondrocytes were set as communication receivers, and the interaction numbers were output for a heatmap and bubble plot.
Transwell assays for SF
CD142+ and CD142– fibroblasts were used for in vitro experiments. For transwell assays, we used 8.0 μm PET membrane in a 24-well format (Corning) for migration assays and 8.0 μm Matrigel-coated PET membrane in a 24-well format (Corning) for invasion assays. Note that MK571 (Selleck) and H89 (MedChemExpress) were dissolved in DMSO, and when added to the experimental system of DMEM/F-12, the concentration of MK571 was 5 μM, and H89 was 15 μM, and DMSO was lower than 0.1%. We added 1 × 105 fibroblasts and 200 μL DMEM/F-12 (0% FBS, 1% penicillin-streptomycin, and 5 μM MK571 or 15 μM H89 for the specified group) in the upper chamber, and 600 μL DMEM/F-12 (10% FBS, 1% penicillin-streptomycin) in the nether chamber. Twenty-four well plates were placed in a 37 °C incubator for 24 h for migration assay and 48 h for invasion assay.
Cytokines stimulation on CD142– SFs
CD142– SFs were sorted by flow cytometry and planted on 6-well plates with 1 × 105 per well. Each identical group of CD142– SFs was seperately stimulated with IL-1β (novoprotein), TNF-α (novoprotein), IL-17α (novoprotein), TGF-β (novoprotein), and INF-γ (novoprotein), which are dissolved in distilled water and with a working concentration of 10 ng/mL. CD142– SFs were placed in a 37 °C incubator for 96 h, and then collected for flow cytometry, ELISA, and qPCR.
Co-culture of SFs-meniscal cells
Primary human RA meniscal cells were used in this study. We used 0.4 μm PET membrane in a 6-well format (Corning). We added 1 × 105 CD142+ or CD142– fibroblasts, 1 ml DMEM/F-12 (10% FBS, 1% penicillin-streptomycin, and 5 μM MK571 for the certain group) in the upper chamber, 1 × 105 meniscal cells, and 2 mL DMEM/F-12 (10% FBS, 1% penicillin-streptomycin) in the lower chamber. Six well plates were placed in a 37 °C incubator for 72 h; then, meniscal cells were washed with PBS and extracted to total RNA (details written below) for further analysis.
Intracellular cAMP concentration assessment by ELISA
Cells cultured in a 6-well plate were washed with PBS and then added with 500 μL lysis buffer (EZ Bioscience). 50 μL of lysis buffer for each group was fetched for assessment. Human Cyclic Adenosine Monophosphate (cAMP) ELISA kit (JONLNBIO) was used according to product guidance. OD values were obtained at 450 nm, and concentration were converted based on the product’s standard curve.
Mouse OA and RA models establishment
This study does not involve sex-based analysis, and sex was not considered in the study design. Therefore, in order to control for the variable, we used only male mice in this study. The animal study design was reviewed and approved by the Ethics Committee of Sun Yat-sen Memorial Hospital (2020-B0270). All animal experiments in this study abide by the ARRIVE guidelines. Mice were housed under standard laboratory conditions (22 ± 2 °C, 50 ± 10% humidity, 12:12 light-dark cycle) with ad libitum access to a conventional rodent chow (containing ~20% protein, ~5% fat, and ~5% fiber) and autoclaved water. Bedding and cages were changed weekly. The euthanasia method of cervical dislocation in mice was applied. We created OA models by surgically destabilizing the medial meniscus (DMM) in C57BL/6 male mice at 8 weeks of age14. After 8 weeks (16 weeks of age), DMM mice were sacrificed to obtain knee samples. We created an RA model with CIA in DBA/1 male mice48. Mice were then immunized with 200 µg chick type II collagen (CII, Chondrex) emulsified 1:1 in complete Freund’s adjuvant (CFA, Chondrex) at 8 weeks of age, and were boosted 3 weeks later with 200 µg CII emulsified 1:1 in Freund’s incomplete adjuvant (IFA, Chondrex). At 16 weeks of age, CIA mice were sacrificed to obtain knee samples.
Mouse invasive SF arthritis model establishment
Human RA CD142+ and CD142– SF were used to create an invasive synovial fibroblast arthritis model. Living CD142+ and CD142– fibroblasts were first labeled with autofluorescence using CellTrace CFSE dye (Thermo Fisher Scientific), which diffused into cells and bound covalently to intracellular amines, resulting in fluorescent staining (excitation: 488 nm; Ex/Em: 592/571 nm). Resuspended fibroblasts in PBS were then added to the CFSE dye at a working concentration of 5 μM and incubated for 20 min at room temperature, protected from light. Five times the original staining volume of the culture medium was added to the fibroblasts, incubated for 5 min, centrifuged, and resuspended in DMEM/F-12. For intra-articular injection, each knee joint was injected with 4 μl cell suspensions (5 × 104 fibroblasts in DMEM/F-12, without FBS and penicillin-streptomycin), while in the MK571 group, each knee joint was injected 2 μl cell suspensions (5 × 104 fibroblasts in DMEM/F-12, without FBS and penicillin-streptomycin) plus 2 μL of MK571 (10 μM).
DBA/1 male mice were anaesthetised using isoflurane as mentioned above. We made a 1 cm vertical incision above the ligamentum patellae to fully expose it, then used a microinjector at a range of 0–10 μL to fetch cell suspensions. The needle tip was parallel to the platform and pointed at the midpoint of the medial or lateral side of the ligamentum patellae. The knee joint was then penetrated, and cell suspensions were injected until visible swelling was observed on the joint without spillage. The tip of the needle was withdrawn, the penetration point was pressed for 2 min using a cotton ball, and the skin was sutured. Intra-articular injection was administered once a week at 8, 9, 10, and 11 weeks of age. Mice were sacrificed at the age of 12 weeks for paraffin-embedded slices and at 16 weeks for both frozen OCT and paraffin-embedded slices. OCT-embedded slices were stained with DAPI and directly observed under a fluorescence microscope to detect the invasive fibroblasts. OCT and paraffin slices were prepared according to the protocol described above.
Histological analysis of mouse joint slices
The knee and wrist joints of mice were decalcified for 3 weeks with 10% EDTA solution (pH 7.2, Solarbio) and embedded in paraffin. Consecutive slices were made (paraffin in 5 μm thick, OCT in 10 μm thick) in the knee lateral compartment at sagittal view and at wrist coronal view. We used the degeneration score and invasion area to assess the destruction of the mouse meniscus, as discussed above. The Krenn score was calculated to assess synovitis in the mouse knees. To evaluate cartilage damage, the OARSI score was calculated, with a maximum of six scores49. The scores of three slices (one slice after an interval of nine slices) were summed and averaged as one joint, whereas both the left and right joints were summed and averaged as an independent sample.
Quantitative reverse transcription PCR
RNA was isolated from the cell suspensions using an RNA isolation kit (EZ Bioscience) according to the manufacturer’s instructions. cDNA synthesis was performed for all samples (500 ng of RNA was transcribed) using the RT Premix cDNA synthesis kit (Accuracy Biology). Reverse transcription quantitative real-time PCR (qRT-PCR) was performed using the SYBR GreenPro Taq qPCR kit (Accuracy Biology) on a real-time PCR detection system (Roche LightCycler480 II). The gene expression levels were quantified using the 2−ΔΔCt method. Each primer (forward, F; reverse, R) was designed based on the sequences available in the NCBI database (Supplementary Table 2).
Bulk RNA sequencing data
Human RA primary CD142+ and CD142– fibroblasts with three independent samples sorted by flow cytometry from the RA knee synovium obtained by TKA surgery were used to extract RNA. Bulk RNA-seq was performed by NovelBio Bio-Pharm Technology Co., Ltd. Fastp was applied with the default parameters to filter the adaptor sequence and remove low-quality reads. The clean reads were aligned to 9606 (Taxonomy ID) genomes (version: GRCh38) using Hisat2. HTseq was used to calculate the gene counts. Reads/fragments per kilobase million reads (RPKM/FPKM) were used to standardize expression data. We applied the DEseq2 algorithm to filter the differentially expressed genes and then filtered the fold change and FDR under the following criteria: i) log2FC > 0.585 or < -0.585; ii) FDR < 0.05. Volcano plots and heat maps were drawn using R based on the analysis of differentially expressed genes, and the color was determined using the filtering criteria. GO analysis was performed to elucidate the biological implications of unique genes in the significant or representative profiles of the differentially expressed genes in the experiment50. We downloaded from the National Center for Biotechnology Information (NCBI; http://www.ncbi.nlm.nih.gov/), UniProt (http://www.uniprot.org/), and GO (http://www.geneontology.org/) databases. Fisher’s exact test was used to identify significant GO categories, and FDR was used to correct p-values.
Statistical analysis
Statistical analyses were performed as described in each section using GraphPad Prism 9 software. Data are presented as mean ± s.d. from at least three independent experiments. Spearman’s correlation analysis was used to test the correlations between ordinal variables. Differences were considered significant at P < 0.05. Multiple testing corrections were applied where appropriate. All measurements of sample size (n) were taken from distinct biological replicates.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.