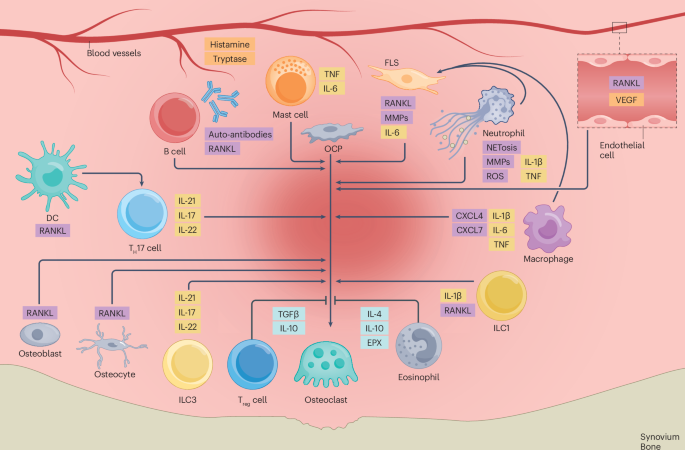
Alivernini, S., Firestein, G. S. & McInnes, I. B. The pathogenesis of rheumatoid arthritis. Immunity 55, 2255–2270 (2022).
Google Scholar
Sims, N. A. & Gooi, J. H. Bone remodeling: multiple cellular interactions required for coupling of bone formation and resorption. Semin. Cell Dev. Biol. 19, 444–451 (2008).
Google Scholar
Martin, T. J. & Ng, K. W. Mechanisms by which cells of the osteoblast lineage control osteoclast formation and activity. J. Cell. Biochem. 56, 357–366 (1994).
Google Scholar
Schett, G. & Teitelbaum, S. L. Osteoclasts and arthritis. J. Bone Miner. Res. 24, 1142–1146 (2009).
Google Scholar
Kim, E. Y. & Moudgil, K. D. Immunomodulation of autoimmune arthritis by pro-inflammatory cytokines. Cytokine 98, 87–96 (2017).
Google Scholar
Andreev, D., Kachler, K., Schett, G. & Bozec, A. Rheumatoid arthritis and osteoimmunology: The adverse impact of a deregulated immune system on bone metabolism. Bone 162, 116468 (2022).
Google Scholar
Hofbauer, L. C. & Heufelder, A. E. Role of receptor activator of nuclear factor-κB ligand and osteoprotegerin in bone cell biology. J. Mol. Med. 79, 243–253 (2001).
Google Scholar
Marques-Carvalho, A., Kim, H.-N. & Almeida, M. The role of reactive oxygen species in bone cell physiology and pathophysiology. Bone Rep. 19, 101664 (2023).
Google Scholar
Bertels, J. C., He, G. & Long, F. Metabolic reprogramming in skeletal cell differentiation. Bone Res. 12, 57 (2024).
Google Scholar
Tsukasaki, M. & Takayanagi, H. Osteoimmunology: evolving concepts in bone–immune interactions in health and disease. Nat. Rev. Immunol. 19, 626–642 (2019). This article provides a comprehensive review that discusses concepts of osteoimmunology.
Google Scholar
McDonald, M. M. et al. Osteoclasts recycle via osteomorphs during RANKL-stimulated bone resorption. Cell 184, 1330–1347.e13 (2021).
Google Scholar
Walker, D. G. Bone resorption restored in osteopetrotic mice by transplants of normal bone marrow and spleen cells. Science 190, 784–785 (1975).
Google Scholar
Walker, D. G. Osteopetrosis cured by temporary parabiosis. Science 180, 875–875 (1973). This article is one of the earliest studies to show that osteoclasts can develop by fusion of monocytic cells.
Google Scholar
Yahara, Y. et al. Erythromyeloid progenitors give rise to a population of osteoclasts that contribute to bone homeostasis and repair. Nat. Cell Biol. 22, 49–59 (2020).
Google Scholar
Kurihara, N., Chenu, C., Miller, M., Civin, C. & Roodman, G. D. Identification of committed mononuclear precursors for osteoclast-like cells formed in long term human marrow cultures. Endocrinology 126, 2733–2741 (1990).
Google Scholar
Udagawa, N. et al. Origin of osteoclasts: mature monocytes and macrophages are capable of differentiating into osteoclasts under a suitable microenvironment prepared by bone marrow-derived stromal cells. Proc. Natl Acad. Sci. USA 87, 7260–7264 (1990).
Google Scholar
Matsuzaki, K. et al. Osteoclast differentiation factor (ODF) induces osteoclast-like cell formation in human peripheral blood mononuclear cell cultures. Biochem. Biophys. Res. Commun. 246, 199–204 (1998).
Google Scholar
Jacome-Galarza, C. E. et al. Developmental origin, functional maintenance and genetic rescue of osteoclasts. Nature 568, 541–545 (2019).
Google Scholar
Novak, S. et al. Osteoclasts derive predominantly from bone marrow–resident CX3CR1+ precursor cells in homeostasis, whereas circulating CX3CR1+ cells contribute to osteoclast development during fracture repair. J. Immunol. 204, 868–878 (2020).
Google Scholar
Rivollier, A. et al. Immature dendritic cell transdifferentiation into osteoclasts: a novel pathway sustained by the rheumatoid arthritis microenvironment. Blood 104, 4029–4037 (2004).
Google Scholar
Gallois, A. et al. Genome-wide expression analyses establish dendritic cells as a new osteoclast precursor able to generate bone-resorbing cells more efficiently than monocytes. J. Bone Miner. Res. 25, 661–672 (2010).
Google Scholar
Alnaeeli, M., Penninger, J. M. & Teng, Y.-T. A. Immune interactions with CD4+ T Cells promote the development of functional osteoclasts from murine CD11c+ dendritic cells. J. Immunol. 177, 3314–3326 (2006).
Google Scholar
Puchner, A. et al. Bona fide dendritic cells are pivotal precursors for osteoclasts. Ann. Rheum. Dis. 83, 518–528 (2024).
Google Scholar
Liu, Y. C. G. & Teng, A. Y. Distinct cross talk of IL-17 & TGF-β with the immature CD11c+TRAF6(−/−)-null myeloid dendritic cell-derived osteoclast precursor (mDDOCp) may engage signaling toward an alternative pathway of osteoclastogenesis for arthritic bone loss in vivo. Immun. Inflamm. Dis. 12, e1173 (2024).
Google Scholar
Maitra, R. et al. Dendritic cell-mediated in vivo bone resorption. J. Immunol. 185, 1485–1491 (2010).
Google Scholar
Wakkach, A. et al. Bone marrow microenvironment controls the in vivo differentiation of murine dendritic cells into osteoclasts. Blood 112, 5074–5083 (2008).
Google Scholar
Tsukasaki, M. et al. Stepwise cell fate decision pathways during osteoclastogenesis at single-cell resolution. Nat. Metab. 2, 1382–1390 (2020).
Google Scholar
Sims, N. A. & Martin, T. J. Coupling the activities of bone formation and resorption: a multitude of signals within the basic multicellular unit. Bonekey Rep. 3, 481 (2014). This article provides a comprehensive review that summarizes cellular activities and signalling crosstalk in bone remodelling.
Google Scholar
Jacquin, C., Gran, D. E., Lee, S. K., Lorenzo, J. A. & Aguila, H. L. Identification of multiple osteoclast precursor populations in murine bone marrow. J. Bone Miner. Res. 21, 67–77 (2009).
Culemann, S. et al. Locally renewing resident synovial macrophages provide a protective barrier for the joint. Nature 572, 670–675 (2019). This article is a landmark paper showing different macrophage populations in the healthy and inflamed joint.
Google Scholar
Meirow, Y. et al. Specific inflammatory osteoclast precursors induced during chronic inflammation give rise to highly active osteoclasts associated with inflammatory bone loss. Bone Res. 10, 36 (2022).
Google Scholar
Takeyama, N. et al. Selective expansion of the CD14+/CD16bright subpopulation of circulating monocytes in patients with hemophagocytic syndrome. Ann. Hematol. 86, 787–792 (2007).
Google Scholar
Nakashima, T. et al. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat. Med. 17, 1231–1234 (2011).
Google Scholar
Kong, Y.-Y. et al. Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature 402, 304–309 (1999). This article is the first to report that activated T cells can activate osteoclasts during inflammatory conditions.
Google Scholar
Kim, K.-W., Kim, H.-R., Kim, B.-M., Cho, M.-L. & Lee, S.-H. Th17 cytokines regulate osteoclastogenesis in rheumatoid arthritis. Am. J. Pathol. 185, 3011–3024 (2015).
Google Scholar
Danks, L. et al. RANKL expressed on synovial fibroblasts is primarily responsible for bone erosions during joint inflammation. Ann. Rheum. Dis. 75, 1187 (2016). This article shows that fibroblast-like synoviocytes are important contributors to inflammatory bone loss by producing RANKL.
Google Scholar
Takayanagi, H. et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev. Cell 3, 889–901 (2002).
Google Scholar
Kondo, N., Kuroda, T. & Kobayashi, D. Cytokine networks in the pathogenesis of rheumatoid arthritis. Int. J. Mol. Sci. 22, 10922 (2021).
Google Scholar
Koga, T. et al. Costimulatory signals mediated by the ITAM motif cooperate with RANKL for bone homeostasis. Nature 428, 758–763 (2004).
Google Scholar
Kim, N., Takami, M., Rho, J., Josien, R. & Choi, Y. A novel member of the leukocyte receptor complex regulates osteoclast differentiation. J. Exp. Med. 195, 201–209 (2002).
Google Scholar
Negishi-Koga, T. & Takayanagi, H. Ca2+-NFATc1 signaling is an essential axis of osteoclast differentiation. Immunol. Rev. 231, 241–256 (2009).
Google Scholar
Zheng, H. et al. Recent advances of NFATc1 in rheumatoid arthritis-related bone destruction: mechanisms and potential therapeutic targets. Mol. Med. 30, 20 (2024).
Google Scholar
Yu, T. et al. Klotho upregulates the interaction between RANK and TRAF6 to facilitate RANKL-induced osteoclastogenesis via the NF-κB signaling pathway. Ann. Transl. Med. 9, 1499 (2021).
Google Scholar
Iotsova, V. et al. Osteopetrosis in mice lacking NF-κB1 and NF-κB2. Nat. Med. 3, 1285–1289 (1997).
Google Scholar
Döffinger, R. et al. X-linked anhidrotic ectodermal dysplasia with immunodeficiency is caused by impaired NF-κB signaling. Nat. Genet. 27, 277–285 (2001).
Google Scholar
Lee, K., Seo, I., Choi, M. H. & Jeong, D. Roles of mitogen-activated protein kinases in osteoclast biology. Int. J. Mol. Sci. 19, 3004 (2018).
Google Scholar
Wagner, E. F. Functions of AP1 (Fos/Jun) in bone development. Ann. Rheum. Dis. 61, ii40 (2002).
Google Scholar
Asagiri, M. et al. Autoamplification of NFATc1 expression determines its essential role in bone homeostasis. J. Exp. Med. 202, 1261–1269 (2005).
Google Scholar
Soriano, P., Montgomery, C., Geske, R. & Bradley, A. Targeted disruption of the c-src proto-oncogene leads to osteopetrosis in mice. Cell 64, 693–702 (1991).
Google Scholar
Wong, B. R. et al. TRANCE, a TNF family member, activates Akt/PKB through a signaling complex involving TRAF6 and c-Src. Mol. Cell 4, 1041–1049 (1999).
Google Scholar
Lacey, D. L. et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 93, 165–176 (1998).
Google Scholar
Cawley, K. M. et al. Local production of osteoprotegerin by osteoblasts suppresses bone resorption. Cell Rep. 32, 108052 (2020).
Google Scholar
Crotti, T. N. et al. Receptor activator NF-κB ligand (RANKL) expression in synovial tissue from patients with rheumatoid arthritis, spondyloarthropathy, osteoarthritis, and from normal patients: semiquantitative and quantitative analysis. Ann. Rheum. Dis. 61, 1047 (2002).
Google Scholar
Pettit, A. R., Walsh, N. C., Manning, C., Goldring, S. R. & Gravallese, E. M. RANKL protein is expressed at the pannus–bone interface at sites of articular bone erosion in rheumatoid arthritis. Rheumatology 45, 1068–1076 (2006).
Google Scholar
Cohen, S. B. et al. Denosumab treatment effects on structural damage, bone mineral density, and bone turnover in rheumatoid arthritis: a twelve-month, multicenter, randomized, double-blind, placebo-controlled, phase II clinical trial. Arthritis Rheum. 58, 1299–1309 (2008).
Google Scholar
Pettit, A. R. et al. TRANCE/RANKL knockout mice are protected from bone erosion in a serum transfer model of arthritis. Am. J. Pathol. 159, 1689–1699 (2001).
Google Scholar
Redlich, K. et al. Tumor necrosis factor α-mediated joint destruction is inhibited by targeting osteoclasts with osteoprotegerin. Arthritis Rheum. 46, 785–792 (2002).
Google Scholar
Hansen, M. S. et al. Transcriptional reprogramming during human osteoclast differentiation identifies regulators of osteoclast activity. Bone Res. 12, 5 (2024).
Google Scholar
Omata, Y. et al. Interspecies single cell RNA seq analysis reveals the novel trajectory of osteoclast differentiation and therapeutic targets. J. Bone Miner. Res. 6, e10631 (2022).
Google Scholar
Steeve, K. T., Marc, P., Sandrine, T., Dominique, H. & Yannick, F. IL-6, RANKL, TNF-alpha/IL-1: interrelations in bone resorption pathophysiology. Cytokine Growth Factor Rev. 15, 49–60 (2004).
Google Scholar
Murray, P. J. et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 41, 14–20 (2014).
Google Scholar
Marahleh, A. et al. TNF-α directly enhances osteocyte RANKL expression and promotes osteoclast formation. Front. Immunol. 10, 2925 (2019).
Google Scholar
Lam, J. et al. TNF-α induces osteoclastogenesis by direct stimulation of macrophages exposed to permissive levels of RANK ligand. J. Clin. Investig. 106, 1481–1488 (2000).
Google Scholar
Yokota, K. et al. Combination of tumor necrosis factor α and interleukin-6 induces mouse osteoclast-like cells with bone resorption activity both in vitro and in vivo. Arthritis Rheumatol. 66, 121–129 (2014). This article shows that the pro-inflammatory cytokines, TNF and IL-6, can stimulate osteoclasts in vitro and in vivo.
Google Scholar
Wei, S., Kitaura, H., Zhou, P., Ross, F. P. & Teitelbaum, S. L. IL-1 mediates TNF-induced osteoclastogenesis. J. Clin. Investig. 115, 282–290 (2005).
Google Scholar
Zwerina, J. et al. TNF-induced structural joint damage is mediated by IL-1. Proc. Natl Acad. Sci. USA 104, 11742–11747 (2007).
Google Scholar
Lee, Z. H. et al. IL-1α stimulation of osteoclast survival through the PI 3-kinase/Akt and ERK pathways. J. Biochem. 131, 161–166 (2002).
Google Scholar
Ruscitti, P. et al. The role of IL-1β in the bone loss during rheumatic diseases. Mediat. Inflamm. 2015, 782382 (2015).
Ishimi, Y. et al. IL-6 is produced by osteoblasts and induces bone resorption. J. Immunol. 145, 3297–3303 (1990).
Google Scholar
Strand, V. et al. High levels of interleukin-6 in patients with rheumatoid arthritis are associated with greater improvements in health-related quality of life for sarilumab compared with adalimumab. Arthritis Res. Ther. 22, 250 (2020).
Google Scholar
Sanchez, C., Gabay, O., Salvat, C., Henrotin, Y. E. & Berenbaum, F. Mechanical loading highly increases IL-6 production and decreases OPG expression by osteoblasts. Osteoarthr. Cartil. 17, 473–481 (2009).
Google Scholar
Adam, S. et al. JAK inhibition increases bone mass in steady-state conditions and ameliorates pathological bone loss by stimulating osteoblast function. Sci. Transl. Med. 12, eaay4447 (2020). This article highlights JAK signalling as a crucial pathway for inflammatory bone loss and proposes it as a novel therapeutic pathway.
Google Scholar
Ortmann, R. A., Cheng, T., Visconti, R., Frucht, D. M. & O’Shea, J. J. Janus kinases and signal transducers and activators of transcription: their roles in cytokine signaling, development and immunoregulation. Arthritis Res. Ther. 2, 16 (1999).
Abu-Amer, Y. IL-4 abrogates osteoclastogenesis through STAT6-dependent inhibition of NF-κB. J. Clin. Investig. 107, 1375–1385 (2001).
Google Scholar
Palmqvist, P. et al. Inhibition of hormone and cytokine-stimulated osteoclastogenesis and bone resorption by interleukin-4 and interleukin-13 is associated with increased osteoprotegerin and decreased RANKL and RANK in a STAT6-dependent pathway. J. Biol. Chem. 281, 2414–2429 (2006).
Google Scholar
Cheng, J. et al. Interleukin-4 inhibits RANKL-induced NFATc1 expression via STAT6: a novel mechanism mediating its blockade of osteoclastogenesis. J. Cell. Biochem. 112, 3385–3392 (2011).
Google Scholar
Bendixen, A. C. et al. IL-4 inhibits osteoclast formation through a direct action on osteoclast precursors via peroxisome proliferator-activated receptor γ1. Proc. Natl Acad. Sci. USA 98, 2443–2448 (2001).
Google Scholar
Scott, T. E. et al. IL-4 and IL-13 induce equivalent expression of traditional M2 markers and modulation of reactive oxygen species in human macrophages. Sci. Rep. 13, 19589 (2023).
Google Scholar
Hata, H. et al. Distinct contribution of IL-6, TNF-α, IL-1, and IL-10 to T cell–mediated spontaneous autoimmune arthritis in mice. J. Clin. Investig. 114, 582–588 (2004).
Google Scholar
Evans, K. E. & Fox, S. W. Interleukin-10 inhibits osteoclastogenesis by reducing NFATc1 expression and preventing its translocation to the nucleus. BMC Cell Biol. 8, 4 (2007).
Google Scholar
Carter, N. A., Rosser, E. C. & Mauri, C. Interleukin-10 produced by B cells is crucial for the suppression of Th17/Th1 responses, induction of T regulatory type 1 cells and reduction of collagen-induced arthritis. Arthritis Res. Ther. 14, R32 (2012).
Google Scholar
Meng, X. et al. Hypoxia-inducible factor-1α is a critical transcription factor for IL-10-producing B cells in autoimmune disease. Nat. Commun. 9, 251 (2018).
Google Scholar
Sapra, L. et al. Regulatory B cells (Bregs) inhibit osteoclastogenesis and play a potential role in ameliorating ovariectomy-induced bone loss. Front. Immunol. 12, 691081 (2021).
Google Scholar
Lee, B., Oh, Y., Jo, S., Kim, T.-H. & Ji, J. D. A dual role of TGF-β in human osteoclast differentiation mediated by Smad1 versus Smad3 signaling. Immunol. Lett. 206, 33–40 (2019).
Google Scholar
Xia, Y. et al. TGFβ reprograms TNF stimulation of macrophages towards a non-canonical pathway driving inflammatory osteoclastogenesis. Nat. Commun. 13, 3920 (2022).
Google Scholar
AlQranei, M. S., Senbanjo, L. T., Aljohani, H., Hamza, T. & Chellaiah, M. A. Lipopolysaccharide- TLR-4 axis regulates osteoclastogenesis independent of RANKL/RANK signaling. BMC Immunol. 22, 23 (2021).
Google Scholar
Weivoda, M. M. & Bradley, E. W. Macrophages and bone remodeling. J. Bone Min. Res. https://doi.org/10.1002/jbmr.4773 (2023).
Google Scholar
Hanlon, M. M. et al. Loss of synovial tissue macrophage homeostasis precedes rheumatoid arthritis clinical onset. Sci. Adv. 10, eadj1252 (2024).
Google Scholar
Wu, D. et al. Synovial macrophages drive severe joint destruction in established rheumatoid arthritis. Sci. Rep. 15, 12111 (2025).
Google Scholar
Chang, M. K. et al. Osteal tissue macrophages are intercalated throughout human and mouse bone lining tissues and regulate osteoblast function in vitro and in vivo. J. Immunol. 181, 1232–1244 (2008).
Google Scholar
Batoon, L. et al. Osteal macrophages support osteoclast-mediated resorption and contribute to bone pathology in a postmenopausal osteoporosis mouse model. J. Bone Miner. Res. 36, 2214–2228 (2021).
Google Scholar
Isojima, T. et al. Bone marrow neutrophil progenitors suppress osteoclast formation in murine cortical and trabecular bone. Blood 146, 1331–1345 (2025).
Google Scholar
O’Neil, L. J. et al. Neutrophil-mediated carbamylation promotes articular damage in rheumatoid arthritis. Sci. Adv. 6, eabd2688 (2020).
Google Scholar
Liu, Y. et al. Neutrophils inhibit bone formation by directly contacting osteoblasts and suppressing osteogenic differentiation. Bone 190, 117310 (2025).
Google Scholar
Herrero-Cervera, A., Soehnlein, O. & Kenne, E. Neutrophils in chronic inflammatory diseases. Cell. Mol. Immunol. 19, 177–191 (2022).
Google Scholar
Li, J. et al. TGFβ1+CCR5+ neutrophil subset increases in bone marrow and causes age-related osteoporosis in male mice. Nat. Commun. 14, 159 (2023).
Google Scholar
Schauer, C. et al. Aggregated neutrophil extracellular traps limit inflammation by degrading cytokines and chemokines. Nat. Med. 20, 511–517 (2014).
Google Scholar
Numazaki, K. et al. Neutrophil extracellular traps inhibit osteoclastogenesis. Biochem. Biophys. Res. Commun. 705, 149743 (2024).
Google Scholar
Khandpur, R. et al. NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Sci. Transl. Med. 5, 178ra40 (2013).
Google Scholar
Gazzinelli-Guimaraes, P. H., Jones, S. M., Voehringer, D., Mayer-Barber, K. D. & Samarasinghe, A. E. Eosinophils as modulators of host defense during parasitic, fungal, bacterial, and viral infections. J. Leukoc. Biol. 116, 1301–1323 (2024).
Google Scholar
Andreev, D. et al. Eosinophils preserve bone homeostasis by inhibiting excessive osteoclast formation and activity via eosinophil peroxidase. Nat. Commun. 15, 1067 (2024).
Google Scholar
Wang, H. et al. Osteoclasts and osteoarthritis: novel intervention targets and therapeutic potentials during aging. Aging Cell 23, e14092 (2024).
Google Scholar
Andreev, D. et al. Regulatory eosinophils induce the resolution of experimental arthritis and appear in remission state of human rheumatoid arthritis. Ann. Rheum. Dis. 80, 451–468 (2021).
Google Scholar
Chen, Z. et al. Th2 and eosinophil responses suppress inflammatory arthritis. Nat. Commun. 7, 11596 (2016).
Google Scholar
Xiao, Y. et al. Macrophages and osteoclasts stem from a bipotent progenitor downstream of a macrophage/osteoclast/dendritic cell progenitor. Blood Adv. 1, 1993–2006 (2017).
Google Scholar
Ng, C. W. et al. Human mast cells induce osteoclastogenesis through cell surface RANKL. Inflamm. Res. 71, 1261–1270 (2022).
Google Scholar
Wang, R., Jaw, J. J., Stutzman, N. C., Zou, Z. & Sun, P. D. Natural killer cell-produced IFN-γ and TNF-α induce target cell cytolysis through up-regulation of ICAM-1. J. Leukoc. Biol. 91, 299–309 (2011).
Google Scholar
Cheng, J. et al. Molecular mechanisms of the biphasic effects of interferon-γ on osteoclastogenesis. J. Interf. Cytokine Res. 32, 34–45 (2011).
Google Scholar
Feng, S. et al. Interleukin-15-activated natural killer cells kill autologous osteoclasts via LFA-1, DNAM-1 and TRAIL, and inhibit osteoclast-mediated bone erosion in vitro. Immunology 145, 367–379 (2015).
Google Scholar
Rauber, S. et al. Resolution of inflammation by interleukin-9-producing type 2 innate lymphoid cells. Nat. Med. 23, 938–944 (2017).
Google Scholar
Omata, Y. et al. Group 2 innate lymphoid cells attenuate inflammatory arthritis and protect from bone destruction in mice. Cell Rep. 24, 169–180 (2018).
Google Scholar
Omata, Y. et al. Type 2 innate lymphoid cells inhibit the differentiation of osteoclasts and protect from ovariectomy-induced bone loss. Bone 136, 115335 (2020).
Google Scholar
Stark, M. A. et al. Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity 22, 285–294 (2005).
Google Scholar
Takaki-Kuwahara, A. et al. CCR6+ group 3 innate lymphoid cells accumulate in inflamed joints in rheumatoid arthritis and produce Th17 cytokines. Arthritis Res. Ther. 21, 198 (2019).
Google Scholar
Takayanagi, H. et al. T-cell-mediated regulation of osteoclastogenesis by signalling cross-talk between RANKL and IFN-γ. Nature 408, 600–605 (2000).
Google Scholar
Levescot, A. et al. IL-1β-driven osteoclastogenic T regulatory cells accelerate bone erosion in arthritis. J. Clin. Investig. 131, e141008 (2021).
Google Scholar
Sato, K. & Takayanagi, H. Osteoclasts, rheumatoid arthritis, and osteoimmunology. Curr. Opin. Rheumatol. 18, 419–426 (2006).
Google Scholar
Kotake, S. et al. IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J. Clin. Investig. 103, 1345–1352 (1999).
Google Scholar
Lubberts, E. The IL-23–IL-17 axis in inflammatory arthritis. Nat. Rev. Rheumatol. 11, 415–429 (2015).
Google Scholar
Huang, H. et al. IL-17 stimulates the proliferation and differentiation of human mesenchymal stem cells: implications for bone remodeling. Cell Death Differ. 16, 1332–1343 (2009).
Google Scholar
Zwerina, K. et al. Anti IL-17A therapy inhibits bone loss in TNF-α-mediated murine arthritis by modulation of the T-cell balance. Eur. J. Immunol. 42, 413–423 (2012).
Google Scholar
Ibáñez, L. et al. Inflammatory osteoclasts prime TNFα-producing CD4+ T cells and express CX3CR1. J. Bone Miner. Res. 31, 1899–1908 (2016).
Google Scholar
Valencia, X. et al. TNF downmodulates the function of human CD4+CD25hi T-regulatory cells. Blood 108, 253–261 (2006).
Google Scholar
Bozec, A. & Zaiss, M. M. T regulatory cells in bone remodelling. Curr. Osteoporos. Rep. 15, 121–125 (2017).
Google Scholar
Zaiss, M. M. et al. Treg cells suppress osteoclast formation: a new link between the immune system and bone. Arthritis Rheum. 56, 4104–4112 (2007).
Google Scholar
Bozec, A. et al. T cell costimulation molecules CD80/86 inhibit osteoclast differentiation by inducing the IDO/tryptophan pathway. Sci. Transl. Med. 6, 235ra60 (2014).
Google Scholar
Buchwald, Z. S., Kiesel, J. R., DiPaolo, R., Pagadala, M. S. & Aurora, R. Osteoclast activated FoxP3+CD8+ T-cells suppress bone resorption in vitro. PLoS One 7, e38199 (2012).
Google Scholar
Alvarez, C. et al. Regulatory T cell phenotype and anti-osteoclastogenic function in experimental periodontitis. Sci. Rep. 10, 19018 (2020).
Google Scholar
Schett, G. The role of ACPAs in at-risk individuals: early targeting of the bone and joints. Best Pract. Res. Clin. Rheumatol. 31, 53–58 (2017).
Google Scholar
Catrina, A., Krishnamurthy, A. & Rethi, B. Current view on the pathogenic role of anti-citrullinated protein antibodies in rheumatoid arthritis. RMD Open 7, e001228 (2021).
Google Scholar
Harre, U. et al. Induction of osteoclastogenesis and bone loss by human autoantibodies against citrullinated vimentin. J. Clin. Investig. 122, 1791–1802 (2012). This article describes the ability of auto-antibodies to modulate osteoclastogenesis.
Google Scholar
Sun, M. et al. Anticitrullinated protein antibodies facilitate migration of synovial tissue-derived fibroblasts. Ann. Rheum. Dis. 78, 1621–1631 (2019).
Google Scholar
Laurent, L. et al. IgM rheumatoid factor amplifies the inflammatory response of macrophages induced by the rheumatoid arthritis-specific immune complexes containing anticitrullinated protein antibodies. Ann. Rheum. Dis. 74, 1425 (2015).
Google Scholar
Seeling, M. et al. Inflammatory monocytes and Fcγ receptor IV on osteoclasts are critical for bone destruction during inflammatory arthritis in mice. Proc. Natl Acad. Sci. USA 110, 10729–10734 (2013).
Google Scholar
Seeling, M. et al. Immunoglobulin G-dependent inhibition of inflammatory bone remodeling requires pattern recognition receptor Dectin-1. Immunity 56, 1046–1063.e7 (2023).
Google Scholar
Ota, Y. et al. Generation mechanism of RANKL+ effector memory B cells: relevance to the pathogenesis of rheumatoid arthritis. Arthritis Res. Ther. 18, 67 (2016).
Google Scholar
Meednu, N. et al. Production of RANKL by memory B cells: a link between B cells and bone erosion in rheumatoid arthritis. Arthritis Rheumatol. 68, 805–816 (2016).
Google Scholar
Meng, X. et al. Estrogen-mediated downregulation of HIF-1α signaling in B lymphocytes influences postmenopausal bone loss. Bone Res. 10, 15 (2022).
Google Scholar
Yeo, L. et al. Cytokine mRNA profiling identifies B cells as a major source of RANKL in rheumatoid arthritis. Ann. Rheum. Dis. 70, 2022 (2011).
Google Scholar
Komatsu, N. et al. Plasma cells promote osteoclastogenesis and periarticular bone loss in autoimmune arthritis. J. Clin. Investig. 131, e143060 (2021).
Google Scholar
Yeo, L. et al. Expression of FcRL4 defines a pro-inflammatory, RANKL-producing B cell subset in rheumatoid arthritis. Ann. Rheum. Dis. 74, 928 (2015).
Google Scholar
Li, Y., Terauchi, M., Vikulina, T., Roser-Page, S. & Weitzmann, M. N. B cell production of both OPG and RANKL is significantly increased in aged mice. Open Bone J. 6, 8–17 (2014).
Google Scholar
Croft, A. P. et al. Distinct fibroblast subsets drive inflammation and damage in arthritis. Nature 570, 246–251 (2019). This article refines the fibroblast subsets that support inflammation-induced osteoclastogenesis in arthritis.
Google Scholar
Mizoguchi, F. et al. Functionally distinct disease-associated fibroblast subsets in rheumatoid arthritis. Nat. Commun. 9, 789 (2018).
Google Scholar
Zhang, F. et al. Defining inflammatory cell states in rheumatoid arthritis joint synovial tissues by integrating single-cell transcriptomics and mass cytometry. Nat. Immunol. 20, 928–942 (2019). This article provides a comprehensive analysis of cell types in the inflamed joint describing, for example, that IL-6 is mainly produced by synovial fibroblasts and IL-1 by monocytes.
Google Scholar
Sawa, S. et al. Autoimmune arthritis associated with mutated interleukin (IL)-6 receptor gp130 is driven by STAT3/IL-7–dependent homeostatic proliferation of CD4+ T cells. J. Exp. Med. 203, 1459–1470 (2006).
Google Scholar
Komatsu, N. & Takayanagi, H. Mechanisms of joint destruction in rheumatoid arthritis — immune cell–fibroblast–bone interactions. Nat. Rev. Rheumatol. 18, 415–429 (2022).
Google Scholar
Liang, Z. et al. Evaluation of the immune feature of ACPA-negative rheumatoid arthritis and the clinical value of matrix metalloproteinase-3. Front. Immunol. 13, 939265 (2022).
Google Scholar
Ishikawa, T. et al. Prevention of progressive joint destruction in adjuvant induced arthritis in rats by a novel matrix metalloproteinase inhibitor, FR217840. Eur. J. Pharmacol. 508, 239–247 (2005).
Google Scholar
Tada, M. et al. Itaconate reduces proliferation and migration of fibroblast-like synoviocytes and ameliorates arthritis models. Clin. Immunol. 264, 110255 (2024).
Google Scholar
Intemann, J., Gorter, D. J. J. D., Naylor, A. J., Dankbar, B. & Wehmeyer, C. Importance of osteocyte-mediated regulation of bone remodelling in inflammatory bone disease. Swiss Med. Wkly 150, w20187 (2020).
Google Scholar
Malysheva, K. et al. Interleukin 6/Wnt interactions in rheumatoid arthritis: interleukin 6 inhibits Wnt signaling in synovial fibroblasts and osteoblasts. Croat. Med. J. 57, 89–98 (2016).
Google Scholar
Diarra, D. et al. Dickkopf-1 is a master regulator of joint remodeling. Nat. Med. 13, 156–163 (2007).
Google Scholar
Glass, D. A. et al. Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev. Cell 8, 751–764 (2005).
Google Scholar
Zheng, L. et al. Dickkopf-1 perpetuated synovial fibroblast activation and synovial angiogenesis in rheumatoid arthritis. Clin. Rheumatol. 40, 4279–4288 (2021).
Google Scholar
Rauner, M. et al. WNT5A is induced by inflammatory mediators in bone marrow stromal cells and regulates cytokine and chemokine production. J. Bone Miner. Res. 27, 575–585 (2011).
Mueller, A. A. et al. Wnt signaling drives stromal inflammation in inflammatory arthritis. Preprint at bioRxiv https://doi.org/10.1101/2025.01.06.631510 (2025).
Menghini, R. et al. Toll-Like receptor 4 mediates endothelial cell activation through NF-κB but is not associated with endothelial dysfunction in patients with rheumatoid arthritis. PLoS One 9, e99053 (2014).
Google Scholar
Buul, J. D. van et al. ICAM-1 clustering on endothelial cells recruits VCAM-1. BioMed. Res. Int. 2010, 120328 (2010).
Koch, A. E. et al. Vascular endothelial growth factor. A cytokine modulating endothelial function in rheumatoid arthritis. J. Immunol. 152, 4149–4156 (1994).
Google Scholar
Ballara, S. et al. Raised serum vascular endothelial growth factor levels are associated with destructive change in inflammatory arthritis. Arthritis Rheum. 44, 2055–2064 (2001).
Google Scholar
Taylor, P. C. Serum vascular markers and vascular imaging in assessment of rheumatoid arthritis disease activity and response to therapy. Rheumatology 44, 721–728 (2005).
Google Scholar
Kim, H.-R., Kim, K.-W., Kim, B.-M., Cho, M.-L. & Lee, S.-H. The effect of vascular endothelial growth factor on osteoclastogenesis in rheumatoid arthritis. PLoS One 10, e0124909 (2015).
Google Scholar
Wang, C. et al. CD147 induces angiogenesis through a vascular endothelial growth factor and hypoxia-inducible transcription factor 1α-mediated pathway in rheumatoid arthritis. Arthritis Rheum. 64, 1818–1827 (2012).
Google Scholar
Zittermann, S. I. & Issekutz, A. C. Endothelial growth factors VEGF and bFGF differentially enhance monocyte and neutrophil recruitment to inflammation. J. Leukoc. Biol. 80, 247–257 (2006).
Google Scholar
Miotla, J., Maciewicz, R., Kendrew, J., Feldmann, M. & Paleolog, E. Treatment with soluble VEGF receptor reduces disease severity in murine collagen-induced arthritis. Lab. Investig. 80, 1195–1205 (2000).
Google Scholar
Wculek, S. K., Dunphy, G., Heras-Murillo, I., Mastrangelo, A. & Sancho, D. Metabolism of tissue macrophages in homeostasis and pathology. Cell. Mol. Immunol. 19, 384–408 (2022).
Google Scholar
Taubmann, J. et al. Metabolic reprogramming of osteoclasts represents a therapeutic target during the treatment of osteoporosis. Sci. Rep. 10, 21020 (2020).
Google Scholar
Song, C. et al. Sexual dimorphism of osteoclast reliance on mitochondrial oxidation of energy substrates in the mouse. JCI Insight 8, e174293 (2023).
Google Scholar
Indo, Y. et al. Metabolic regulation of osteoclast differentiation and function. J. Bone Miner. Res. 28, 2392–2399 (2013).
Google Scholar
Ledesma-Colunga, M. G., Passin, V., Lademann, F., Hofbauer, L. C. & Rauner, M. Novel insights into osteoclast energy metabolism. Curr. Osteoporos. Rep. 21, 660–669 (2023).
Google Scholar
Arisumi, S. et al. Metallothionein 3 promotes osteoclast differentiation and survival by regulating the intracellular Zn2+ concentration and NRF2 pathway. Cell Death Discov. 9, 436 (2023).
Google Scholar
Ha, H. et al. Reactive oxygen species mediate RANK signaling in osteoclasts. Exp. Cell Res. 301, 119–127 (2004).
Google Scholar
Peace, C. G. & O’Neill, L. A. J. The role of itaconate in host defense and inflammation. J. Clin. Investig. 132, e148548 (2022).
Google Scholar
Lampropoulou, V. et al. Itaconate links inhibition of succinate dehydrogenase with macrophage metabolic remodeling and regulation of inflammation. Cell Metab. 24, 158–166 (2016).
Google Scholar
Kachler, K. et al. Acod1-mediated inhibition of aerobic glycolysis suppresses osteoclast differentiation and attenuates bone erosion in arthritis. Ann. Rheum. Dis. 83, e224774 (2024).
Google Scholar
Takarada, T. et al. Osteoclastogenesis is negatively regulated by D-serine produced by osteoblasts. J. Cell. Physiol. 227, 3477–3487 (2012).
Google Scholar
Karkache, I. Y., Damodaran, J. R., Molstad, D. H. H. & Bradley, E. W. Serine/threonine phosphatases in osteoclastogenesis and bone resorption. Gene 771, 145362 (2021).
Google Scholar
Stegen, S., Moermans, K., Stockmans, I., Thienpont, B. & Carmeliet, G. The serine synthesis pathway drives osteoclast differentiation through epigenetic regulation of NFATc1 expression. Nat. Metab. 6, 141–152 (2024).
Google Scholar
Brunner, J. S. et al. Environmental arginine controls multinuclear giant cell metabolism and formation. Nat. Commun. 11, 431 (2020).
Google Scholar
Liu, H. & Rosen, C. J. Nitric oxide and bone: the phoenix rises again. J. Clin. Investig. 131, e147072 (2021).
Google Scholar
Kilic, G. & Ozgocmen, S. Hand bone mass in rheumatoid arthritis: a review of the literature. World J. Orthop. 6, 106–116 (2015).
Google Scholar
Nagaraj, S., Finzel, S., Stok, K. S., Barnabe, C. & SPECTRA Collaboration. High-resolution Peripheral quantitative computed tomography imaging in the assessment of periarticular bone of metacarpophalangeal and wrist joints. J. Rheumatol. 43, 1921–1934 (2016).
Google Scholar
Kleyer, A. et al. Bone loss before the clinical onset of rheumatoid arthritis in subjects with anticitrullinated protein antibodies. Ann. Rheum. Dis. 73, 854 (2014).
Google Scholar
Kapetanovic, M. C. et al. Early changes in bone mineral density measured by digital X-ray radiogrammetry predict up to 20 years radiological outcome in rheumatoid arthritis. Arthritis Res. Ther. 13, R31 (2011).
Google Scholar
Grossman, J. M. et al. American College of Rheumatology 2010 recommendations for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Care Res. 62, 1515–1526 (2010).
Steinbuch, M., Youket, T. E. & Cohen, S. Oral glucocorticoid use is associated with an increased risk of fracture. Osteoporos. Int. 15, 323–328 (2004).
Google Scholar
Motavalli, R. et al. The clinical significance of the glucocorticoid receptors: genetics and epigenetics. J. Steroid Biochem. Mol. Biol. 213, 105952 (2021).
Google Scholar
Hofbauer, L. C. et al. Stimulation of osteoprotegerin ligand and inhibition of osteoprotegerin production by glucocorticoids in human osteoblastic lineage cells: potential paracrine mechanisms of glucocorticoid-induced osteoporosis1. Endocrinology 140, 4382–4389 (1999). This article shows that glucocorticoids induce RANKL and thus osteoclastogenesis.
Google Scholar
Thiele, S. et al. Selective glucocorticoid receptor modulation maintains bone mineral density in mice. J. Bone Miner. Res. 27, 2242–2250 (2012).
Google Scholar
O’Brien, C. A. et al. Glucocorticoids act directly on osteoblasts and osteocytes to induce their apoptosis and reduce bone formation and strength. Endocrinology 145, 1835–1841 (2004).
Google Scholar
Rauch, A. et al. Glucocorticoids suppress bone formation by attenuating osteoblast differentiation via the monomeric glucocorticoid receptor. Cell Metab. 11, 517–531 (2010).
Google Scholar
Colditz, J. et al. Osteogenic Dkk1 mediates glucocorticoid-induced but not arthritis-induced bone loss. J. Bone Miner. Res. 34, 1314–1323 (2019).
Google Scholar
Sato, A. Y. et al. Protection from glucocorticoid-induced osteoporosis by anti-catabolic signaling in the absence of Sost/Sclerostin. J. Bone Miner. Res. 31, 1791–1802 (2016).
Google Scholar
Kanagawa, H. et al. Methotrexate inhibits osteoclastogenesis by decreasing RANKL-induced calcium influx into osteoclast progenitors. J. Bone Miner. Metab. 34, 526–531 (2016).
Google Scholar
Revu, S., Neregård, P., Klint, E., Korotkova, M. & Catrina, A. I. Synovial membrane immunohistology in early-untreated rheumatoid arthritis reveals high expression of catabolic bone markers that is modulated by methotrexate. Arthritis Res. Ther. 15, R205 (2013).
Google Scholar
Hensvold, A. H. et al. Serum RANKL levels associate with anti-citrullinated protein antibodies in early untreated rheumatoid arthritis and are modulated following methotrexate. Arthritis Res. Ther. 17, 239 (2015).
Google Scholar
Fan, C. et al. Damaging effects of chronic low-dose methotrexate usage on primary bone formation in young rats and potential protective effects of folinic acid supplementary treatment. Bone 44, 61–70 (2009).
Google Scholar
May, K. P., West, S. G., Mcdermott, M. T. & Huffer, W. E. The effect of low-dose methotrexate on bone metabolism and histomorphometry in rats. Arthritis Rheum. 37, 201–206 (1994).
Google Scholar
May, K. P., Mercill, D., McDermott, M. T. & West, S. G. The effect of methotrexate on mouse bone cells in culture. Arthritis Rheum. 39, 489–494 (1996).
Google Scholar
Wheeler, D. L. et al. The short- and long-term effects of methotrexate on the rat skeleton. Bone 16, 215–221 (1995).
Google Scholar
Xian, C. J. et al. Cellular mechanisms for methotrexate chemotherapy-induced bone growth defects. Bone 41, 842–850 (2007).
Google Scholar
Tronstad, I. et al. Rheumatoid arthritis, disease-modifying antirheumatic drugs and risk of major osteoporotic fracture: prospective data from the HUNT Study, Norway. RMD Open 10, e003919 (2024).
Google Scholar
Minaur, N. J. et al. Methotrexate in the treatment of rheumatoid arthritis. II. In vivo effects on bone mineral density. Rheumatology 41, 741–749 (2002).
Google Scholar
Rolvien, T. et al. Clinical and radiological characterization of patients with immobilizing and progressive stress fractures in methotrexate osteopathy. Calcif. Tissue Int. 108, 219–230 (2021).
Google Scholar
Robin, F. et al. Methotrexate osteopathy: five cases and systematic literature review. Osteoporos. Int. 32, 225–232 (2021).
Google Scholar
Brackel, F. N., von, Grambeck, J., Barvencik, F., Amling, M. & Oheim, R. MTX osteopathy versus osteoporosis including response to treatment data — a retrospective single center study including 172 patients. Calcif. Tissue Int. 115, 599–610 (2024).
Lee, C. et al. Effects of disease-modifying antirheumatic drugs and antiinflammatory cytokines on human osteoclastogenesis through interaction with receptor activator of nuclear factor κB, osteoprotegerin, and receptor activator of nuclear factor κB ligand. Arthritis Rheum. 50, 3831–3843 (2004).
Google Scholar
Suematsu, A. et al. Scientific basis for the efficacy of combined use of antirheumatic drugs against bone destruction in rheumatoid arthritis. Mod. Rheumatol. 17, 17–23 (2007).
Google Scholar
Kobayashi, Y. et al. The active metabolite of leflunomide, A771726, inhibits both the generation of and the bone-resorbing activity of osteoclasts by acting directly on cells of the osteoclast lineage. J. Bone Miner. Metab. 22, 318–328 (2004).
Google Scholar
Rexhepi, S., Rexhepi, M., Sahatçiu-Meka, V., Mahmutaj, V. & Boshnjaku, S. The impact of low-dose disease-modifying anti-rheumatics drugs (DMARDs) on bone mineral density of premenopausal women in early rheumatoid arthritis. Med. Arch. 70, 101 (2016).
Google Scholar
Pfeil, A. et al. Effects of leflunomide and methotrexate in rheumatoid arthritis detected by digital X-ray radiogrammetry and computer-aided joint space analysis. Rheumatol. Int. 29, 287–295 (2009).
Google Scholar
Schett, G., Elewaut, D., McInnes, I. B., Dayer, J.-M. & Neurath, M. F. How cytokine networks fuel inflammation: toward a cytokine-based disease taxonomy. Nat. Med. 19, 822–824 (2013).
Google Scholar
Marotte, H. et al. A 1-year case-control study in patients with rheumatoid arthritis indicates prevention of loss of bone mineral density in both responders and nonresponders to infliximab. Arthritis Res. Ther. 9, R61 (2007).
Google Scholar
Vis, M. et al. Evaluation of bone mineral density, bone metabolism, osteoprotegerin and receptor activator of the NFκB ligand serum levels during treatment with infliximab in patients with rheumatoid arthritis. Ann. Rheum. Dis. 65, 1495 (2006).
Google Scholar
Lange, U., Teichmann, J., Müller-Ladner, U. & Strunk, J. Increase in bone mineral density of patients with rheumatoid arthritis treated with anti-TNF-α antibody: a prospective open-label pilot study. Rheumatology 44, 1546–1548 (2005). This article shows that anti-TNF therapy is osteoprotective in arthritis.
Google Scholar
Wijbrandts, C. A. et al. Bone mineral density in rheumatoid arthritis patients 1 year after adalimumab therapy: arrest of bone loss. Ann. Rheum. Dis. 68, 373 (2009).
Google Scholar
Krieckaert, C. L. M., Nurmohamed, M. T., Wolbink, G. & Lems, W. F. Changes in bone mineral density during long-term treatment with adalimumab in patients with rheumatoid arthritis: a cohort study. Rheumatology 52, 547–553 (2013).
Google Scholar
Chopin, F. et al. Long-term effects of infliximab on bone and cartilage turnover markers in patients with rheumatoid arthritis. Ann. Rheum. Dis. 67, 353–357 (2008).
Google Scholar
Eekman, D. A. et al. Stable bone mineral density in lumbar spine and hip in contrast to bone loss in the hands during long-term treatment with infliximab in patients with rheumatoid arthritis. Ann. Rheum. Dis. 70, 389 (2011).
Google Scholar
Hoff, M. et al. Adalimumab reduces hand bone loss in rheumatoid arthritis independent of clinical response: subanalysis of the PREMIER study. BMC Musculoskelet. Disord. 12, 54 (2011).
Google Scholar
Finzel, S. et al. Repair of bone erosions in rheumatoid arthritis treated with tumour necrosis factor inhibitors is based on bone apposition at the base of the erosion. Ann. Rheum. Dis. 70, 1587 (2011).
Google Scholar
Lukas, C., Heijde, D., van der, Fatenajad, S. & Landewé, R. Repair of erosions occurs almost exclusively in damaged joints without swelling. Ann. Rheum. Dis. 69, 851 (2010).
Google Scholar
Saidenberg-Kermanac’h, N. et al. TNF-α antibodies and osteoprotegerin decrease systemic bone loss associated with inflammation through distinct mechanisms in collagen-induced arthritis. Bone 35, 1200–1207 (2004).
Google Scholar
Abtahi, S. et al. Biological disease-modifying antirheumatic drugs and osteoporotic fracture risk in patients with rheumatoid arthritis: a Danish cohort study. Am. J. Med. 135, 879–888.e3 (2022).
Google Scholar
Kim, S. Y., Schneeweiss, S., Liu, J. & Solomon, D. H. Effects of disease-modifying antirheumatic drugs on nonvertebral fracture risk in rheumatoid arthritis: a population-based cohort study. J. Bone Miner. Res. 27, 789–796 (2012).
Google Scholar
Kawai, V. K. et al. Initiation of tumor necrosis factor α antagonists and risk of fractures in patients with selected rheumatic and autoimmune diseases. Arthritis Care Res. 65, 1085–1094 (2013).
Google Scholar
Coulson, K. A., Reed, G., Gilliam, B. E., Kremer, J. M. & Pepmueller, P. H. Factors influencing fracture risk, T score, and management of osteoporosis in patients with rheumatoid arthritis in the Consortium of Rheumatology Researchers of North America (CORRONA) registry. J. Clin. Rheumatol. 15, 155–160 (2009).
Google Scholar
Ozen, G., Pedro, S., Wolfe, F. & Michaud, K. Medications associated with fracture risk in patients with rheumatoid arthritis. Ann. Rheum. Dis. 78, 1041–1047 (2019).
Google Scholar
Shao, F., Li, H.-C., Wang, M.-J. & Cui, C.-M. Impact of biologic disease-modifying antirheumatic drugs on fracture risk in patients with rheumatoid arthritis: a systematic review and meta-analysis. Eur. Rev. Med. Pharmacol. Sci. 25, 3416–3424 (2021).
Google Scholar
Maini, R. N. et al. Double-blind randomized controlled clinical trial of the interleukin-6 receptor antagonist, tocilizumab, in European patients with rheumatoid arthritis who had an incomplete response to methotrexate. Arthritis Rheum. 54, 2817–2829 (2006).
Google Scholar
Smolen, J. S. et al. Effect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double-blind, placebo-controlled, randomised trial. Lancet 371, 987–997 (2008).
Google Scholar
Wong, P. K. K. et al. Interleukin-6 modulates production of T lymphocyte–derived cytokines in antigen-induced arthritis and drives inflammation-induced osteoclastogenesis. Arthritis Rheum. 54, 158–168 (2006).
Google Scholar
Finzel, S. et al. Comparison of the effects of tocilizumab monotherapy and adalimumab in combination with methotrexate on bone erosion repair in rheumatoid arthritis. Ann. Rheum. Dis. 78, 1186–1191 (2019). This article investigates the effect of cytokine-blockers on bone erosions using high-resolution peripheral quantitative computed tomography.
Google Scholar
Nishimoto, N. et al. Study of active controlled monotherapy used for rheumatoid arthritis, an IL-6 inhibitor (SAMURAI): evidence of clinical and radiographic benefit from an x ray reader-blinded randomised controlled trial of tocilizumab. Ann. Rheum. Dis. 66, 1162 (2007).
Google Scholar
Kremer, J. M. et al. Tocilizumab inhibits structural joint damage in rheumatoid arthritis patients with inadequate responses to methotrexate: results from the double-blind treatment phase of a randomized placebo-controlled trial of tocilizumab safety and prevention of structural joint damage at one year. Arthritis Rheum. 63, 609–621 (2011).
Google Scholar
Garnero, P., Thompson, E., Woodworth, T. & Smolen, J. S. Rapid and sustained improvement in bone and cartilage turnover markers with the anti-interleukin-6 receptor inhibitor tocilizumab plus methotrexate in rheumatoid arthritis patients with an inadequate response to methotrexate: results from a substudy of the multicenter double-blind, placebo-controlled trial of tocilizumab in inadequate responders to methotrexate alone. Arthritis Rheum. 62, 33–43 (2010).
Google Scholar
Karsdal, M. A. et al. IL-6 receptor inhibition positively modulates bone balance in rheumatoid arthritis patients with an inadequate response to anti-tumor necrosis factor therapy: biochemical marker analysis of bone metabolism in the tocilizumab RADIATE Study (NCT00106522). Semin. Arthritis Rheum. 42, 131–139 (2012).
Google Scholar
Axmann, R. et al. Inhibition of interleukin-6 receptor directly blocks osteoclast formation in vitro and in vivo. Arthritis Rheum. 60, 2747–2756 (2009).
Google Scholar
Kato, A. et al. Early effects of tocilizumab on bone and bone marrow lesions in a collagen-induced arthritis monkey model. Exp. Mol. Pathol. 84, 262–270 (2008).
Google Scholar
Khayyat, S. G. A. et al. Bone-sparing effects of tocilizumab in rheumatoid arthritis: a monocentric observational study. Reumatologia 60, 326–331 (2022).
Google Scholar
Kume, K. et al. The effect of tocilizumab on bone mineral density in patients with methotrexate-resistant active rheumatoid arthritis. Rheumatology 53, 900–903 (2014).
Google Scholar
Briot, K. et al. The effect of tocilizumab on bone mineral density, serum levels of Dickkopf-1 and bone remodeling markers in patients with rheumatoid arthritis. Jt Bone Spine 82, 109–115 (2015).
Google Scholar
Axmann, R. et al. CTLA-4 directly inhibits osteoclast formation. Ann. Rheum. Dis. 67, 1603 (2008).
Google Scholar
Tada, M. et al. FRI0062 Influence of biologic agents on bone mineral density and bone mineral markers in patients with rheumatoid arthritis: data from the airtight study. Ann. Rheum. Dis. 74, 441–442 (2015).
Kremer, J. M. et al. Effects of abatacept in patients with methotrexate-resistant active rheumatoid arthritis: a randomized trial. Ann. Intern. Med. 144, 865–876 (2006).
Google Scholar
Iwamoto, N. et al. Preferable effect of CTLA4-Ig on both bone erosion and bone microarchitecture in rheumatoid arthritis revealed by HR-pQCT. Sci. Rep. 14, 27673 (2024).
Google Scholar
Hein, G. et al. Influence of rituximab on markers of bone remodeling in patients with rheumatoid arthritis: a prospective open-label pilot study. Rheumatol. Int. 31, 269–272 (2011).
Google Scholar
Boumans, M. J. H. et al. Rituximab abrogates joint destruction in rheumatoid arthritis by inhibiting osteoclastogenesis. Ann. Rheum. Dis. 71, 108 (2012).
Google Scholar
Khayyat, S. G. A. et al. Bone-sparing effects of rituximab and body composition analysis in a cohort of postmenopausal women affected by rheumatoid arthritis – retrospective study. Reumatologia 59, 206–210 (2021).
Google Scholar
Zerbini, C. A. & Lomonte, A. B. V. Tofacitinib for the treatment of rheumatoid arthritis. Expert Rev. Clin. Immunol. 8, 319–331 (2012).
Google Scholar
Conaghan, P. G. et al. Comparing the effects of tofacitinib, methotrexate and the combination, on bone marrow oedema, synovitis and bone erosion in methotrexate-naive, early active rheumatoid arthritis: results of an exploratory randomised MRI study incorporating semiquantitative and quantitative techniques. Ann. Rheum. Dis. 75, 1024 (2016).
Google Scholar
Lee, E. B. et al. Tofacitinib versus methotrexate in rheumatoid arthritis. N. Engl. J. Med. 370, 2377–2386 (2014).
Google Scholar
Kremer, J. et al. Tofacitinib in combination with nonbiologic disease-modifying antirheumatic drugs in patients with active rheumatoid arthritis: a randomized trial. Ann. Intern. Med. 159, 253–261 (2013).
Google Scholar
LaBranche, T. P. et al. JAK inhibition with tofacitinib suppresses arthritic joint structural damage through decreased RANKL production. Arthritis Rheum. 64, 3531–3542 (2012).
Google Scholar
Hamar, A. et al. Effects of one-year tofacitinib therapy on bone metabolism in rheumatoid arthritis. Osteoporos. Int. 32, 1621–1629 (2021).
Google Scholar
Hansen, K. E. et al. Fracture in clinical studies of tofacitinib in rheumatoid arthritis. Ther. Adv. Musculoskelet. Dis. 14, 1759720×221142346 (2022).
Google Scholar
Gómez-Vaquero, C. et al. High incidence of clinical fragility fractures in postmenopausal women with rheumatoid arthritis. A case-control study. Bone 168, 116654 (2023).
Google Scholar
Fujieda, Y. et al. Efficacy and safety of sodium RISedronate for glucocorticoid-induced OsTeoporosis with rheumaTOid arthritis (RISOTTO study): a multicentre, double-blind, randomized, placebo-controlled trial. Mod. Rheumatol. 31, 593–599 (2020).
Google Scholar
Kumagai, K. et al. Effects of once-monthly minodronate versus risedronate in osteoporosis patients with rheumatoid arthritis: a 12-month randomized head-to-head comparison. Osteoporos. Int. 29, 1637–1642 (2018).
Google Scholar
Eggelmeijer, F. et al. Increased bone mass with pamidronate treatment in rheumatoid arthritis. Results of a three-year randomized, double-blind trial. Arthritis Rheum. 39, 396–402 (1996).
Google Scholar
Lems, W. F. et al. Positive effect of alendronate on bone mineral density and markers of bone turnover in patients with rheumatoid arthritis on chronic treatment with low-dose prednisone: a randomized, double-blind, placebo-controlled trial. Osteoporos. Int. 17, 716–723 (2006).
Google Scholar
Mawatari, T. et al. Vertebral strength changes in rheumatoid arthritis patients treated with alendronate, as assessed by finite element analysis of clinical computed tomography scans: a prospective randomized clinical trial. Arthritis Rheum. 58, 3340–3349 (2008).
Google Scholar
Katayama, K. & Matsuno, T. Effects of bisphosphonates on fracture incidence and bone metabolism in rheumatoid arthritis patients in general practice taking long-term corticosteroid therapy. Clin. Drug Investig. 28, 149–158 (2008).
Google Scholar
Jarrett, S. J. et al. Preliminary evidence for a structural benefit of the new bisphosphonate zoledronic acid in early rheumatoid arthritis. Arthritis Rheum. 54, 1410–1414 (2006).
Google Scholar
Xie, J. et al. Zoledronic acid ameliorates the effects of secondary osteoporosis in rheumatoid arthritis patients. J. Orthop. Surg. Res. 14, 421 (2019).
Google Scholar
Sansoni, P. et al. Inhibition of antigen-presenting cell function by alendronate in vitro. J. Bone Miner. Res. 10, 1719–1725 (1995).
Google Scholar
D’Amelio, P. et al. Risedronate reduces osteoclast precursors and cytokine production in postmenopausal osteoporotic women. J. Bone Miner. Res. 23, 373–379 (2009).
Sharp, J. T. et al. Denosumab prevents metacarpal shaft cortical bone loss in patients with erosive rheumatoid arthritis. Arthritis Care Res. 62, 537–544 (2010).
Deodhar, A. et al. Denosumab-mediated increase in hand bone mineral density associated with decreased progression of bone erosion in rheumatoid arthritis patients. Arthritis Care Res. 62, 569–574 (2010).
Google Scholar
Dore, R. K. et al. Effects of denosumab on bone mineral density and bone turnover in patients with rheumatoid arthritis receiving concurrent glucocorticoids or bisphosphonates. Ann. Rheum. Dis. 69, 872–875 (2010).
Google Scholar
Ferrari-Lacraz, S. & Ferrari, S. Do RANKL inhibitors (denosumab) affect inflammation and immunity? Osteoporos. Int. 22, 435–446 (2011).
Google Scholar
Curtis, J. R. et al. Risk of hospitalized infection among rheumatoid arthritis patients concurrently treated with a biologic agent and denosumab. Arthritis Rheumatol. 67, 1456–1464 (2015).
Google Scholar
Takeuchi, T. et al. Effects of the anti-RANKL antibody denosumab on joint structural damage in patients with rheumatoid arthritis treated with conventional synthetic disease-modifying antirheumatic drugs (DESIRABLE study): a randomised, double-blind, placebo-controlled phase 3 trial. Ann. Rheum. Dis. 78, 899–907 (2019).
Google Scholar
Neer, R. M. et al. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N. Engl. J. Med. 344, 1434–1441 (2001).
Google Scholar
Langdahl, B. L. et al. Real-world effectiveness of teriparatide on fracture reduction in patients with osteoporosis and comorbidities or risk factors for fractures: Integrated analysis of 4 prospective observational studies. Bone 116, 58–66 (2018).
Google Scholar
Ebina, K. et al. Assessment of the effects of switching oral bisphosphonates to denosumab or daily teriparatide in patients with rheumatoid arthritis. J. Bone Miner. Metab. 36, 478–487 (2018).
Google Scholar
Solomon, D. H. et al. Effects of teriparatide on joint erosions in rheumatoid arthritis: a randomized controlled trial. Arthritis Rheumatol. 69, 1741–1750 (2017).
Google Scholar
Sleeman, A. & Clements, J. N. Abaloparatide: a new pharmacological option for osteoporosis. Am. J. Health Syst. Pharm. 76, 130–135 (2019).
Google Scholar
McClung, M. R. et al. Romosozumab in postmenopausal women with low bone mineral density. N. Engl. J. Med. 370, 412–420 (2014).
Google Scholar
Saag, K. G. et al. Romosozumab or alendronate for fracture prevention in women with osteoporosis. N. Engl. J. Med. 377, 1417–1427 (2017).
Google Scholar
Ebina, K. et al. An investigation of the differential therapeutic effects of romosozumab on postmenopausal osteoporosis patients with or without rheumatoid arthritis complications: a case–control study. Osteoporos. Int. 35, 841–849 (2024).
Google Scholar
Kobayakawa, T. et al. Comparable efficacy of denosumab and romosozumab in patients with rheumatoid arthritis receiving glucocorticoid administration. Mod. Rheumatol. 33, 96–103 (2022).
Mochizuki, T., Yano, K., Ikari, K., Hiroshima, R. & Okazaki, K. Comparison of romosozumab versus denosumab treatment on bone mineral density after 1 year in rheumatoid arthritis patients with severe osteoporosis: a randomized clinical pilot study. Mod. Rheumatol. 33, 490–495 (2022).
Mok, C. C. et al. Romosozumab versus denosumab in long-term users of glucocorticoids: a pilot randomized controlled trial. J. Intern. Med. 296, 481–494 (2024).
Google Scholar
Gordon, D. et al. Selective inhibition of the MK2 pathway: data from a phase IIa randomized clinical trial in rheumatoid arthritis. ACR Open Rheumatol. 5, 63–70 (2023).
Google Scholar
Wehmeyer, C. et al. Sclerostin inhibition promotes TNF-dependent inflammatory joint destruction. Sci. Transl. Med. 8, 330ra35 (2016).
Google Scholar
Chen, Y. et al. A selected small molecule prevents inflammatory osteolysis through restraining osteoclastogenesis by modulating PTEN activity. Clin. Transl. Med. 10, e240 (2020).
Google Scholar
Manda, G. et al. Pros and cons of NRF2 activation as adjunctive therapy in rheumatoid arthritis. Free Radic. Biol. Med. 190, 179–201 (2022).
Google Scholar
Chery, J. RNA therapeutics: RNAi and antisense mechanisms and clinical applications. Postdoc J. 4, 35–50 (2016).
Google Scholar
Hasegawa, T. et al. Identification of a novel arthritis-associated osteoclast precursor macrophage regulated by FoxM1. Nat. Immunol. 20, 1631–1643 (2019). This article describes the occurrence of arthritis-associated osteoclast precursors in mice.
Google Scholar
Yamaguchi, Y. et al. Dimethyl fumarate inhibits osteoclasts via attenuation of reactive oxygen species signalling by augmented antioxidation. J. Cell. Mol. Med. 22, 1138–1147 (2018).
Google Scholar
Cao, S. et al. L-arginine metabolism inhibits arthritis and inflammatory bone loss. Ann. Rheum. Dis. 83, 72–87 (2024).
Google Scholar